MXenes, a class of two-dimensional transition metal carbides and nitrides, have emerged as promising candidates for biomedical applications due to their electrical conductivity, photothermal response, and rich surface chemistry. However, their biocompatibility is highly sensitive to synthesis conditions, particularly etching and delamination strategies. In this study, we systematically investigated the influence of different synthesis routes─using acidic (concentrated or diluted HF/HCl) etching and Li+ versus Na+ intercalation─on the surface chemistry, structural integrity, and biological behavior of Ti3C2Tx and its carbonitride analog Ti3C1.5N0.5Tx.
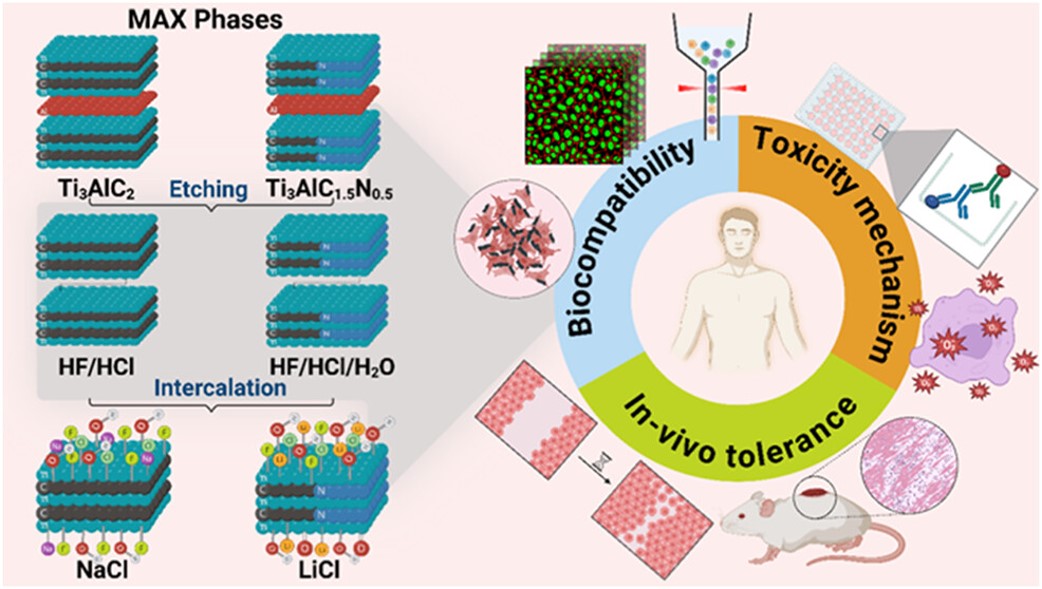
Detailed physicochemical characterization revealed that water-assisted etching and Na+ intercalation enhanced hydroxylation and reduced fluorine terminations. Biological assays using human keratinocytes (HaCaT) demonstrated that Ti3C1.5N0.5Tx exhibited superior biocompatibility compared to Ti3C2Tx, with lower cytotoxicity, diminished ROS generation, minimal inflammatory signaling (IL-6 and IL-8 interleukins), and preserved wound healing capacity. Among Ti3C2Tx variants, the combination of diluted etchant and Na+ intercalation significantly improved biological tolerance, minimizing apoptosis and oxidative stress. These findings underscore the critical role of surface chemistry in MXene-cell interactions and offer a practical guide to engineering safer MXenes for biomedical use.
This study shows that the biocompatibility of Ti₃C₂Tₓ and Ti₃C₁.₅N₀.₅Tₓ MXenes can be finely tuned by adjusting synthesis parameters, particularly etching conditions and intercalant selection. The resulting surface terminations—determined by etchant chemistry and delamination route—play a central role in shaping cytotoxicity, oxidative stress, inflammatory signaling, wound healing, and skin tolerance. Notably, Ti₃C₁.₅N₀.₅Tₓ consistently outperformed Ti₃C₂Tₓ in terms of biological compatibility, while dilute etchants combined with Na⁺ intercalation produced MXenes with fewer −F terminations, greater hydroxylation, and improved cellular responses.
At subcytotoxic concentrations (≤25 μg/mL), these optimized formulations maintained keratinocyte viability, promoted wound closure, and elicited minimal oxidative and inflammatory reactions in vitro. Complementary in vivo histological analyses confirmed the absence of acute skin toxicity across all tested MXenes, with no evidence of tissue damage, immune cell infiltration, or mast cell activation—even in regions containing dermal MXene aggregates. Together, these findings highlight the decisive influence of surface terminations (−F vs. −OH), intercalant residues, particle size, and colloidal stability on MXene–cell interactions. By carefully tuning these parameters, it is possible to engineer MXenes that preserve their functional properties while minimizing cytotoxicity and inflammation. Such tailored MXenes hold strong promise for safe translation into biomedical applications, as demonstrated by the negligible cytotoxicity and lack of acute inflammatory response observed in our most refined samples.
This research was supported by Horizon Europe MSCA-2021-SE-01 projects MX-MAP (#101086184) and ESCULAPE (#101131147).
Read more about this study: Kateryna Diedkova, Iryna Roslyk, Nikola Kanas, Lita Grine, Volodymyr Deineka, Agata Blacha-Grzechnik, Martins Boroduskis, Igor Iatsunskyi, Błażej Anastaziak, Anastasia Konieva, Pavlo Shubin, Wojciech Simka, Marks Truhins, Oksana Sulaieva, Ilya Yanko, Veronika Zahorodna, Goran Stojanovic, Oleksiy Gogotsi, Yury Gogotsi, and Maksym Pogorielov. Effects of Etching and Delamination on Biocompatibility of Ti-Based MXenes. ACS Applied Materials & Interfaces. DOI: 10.1021/acsami.5c08807



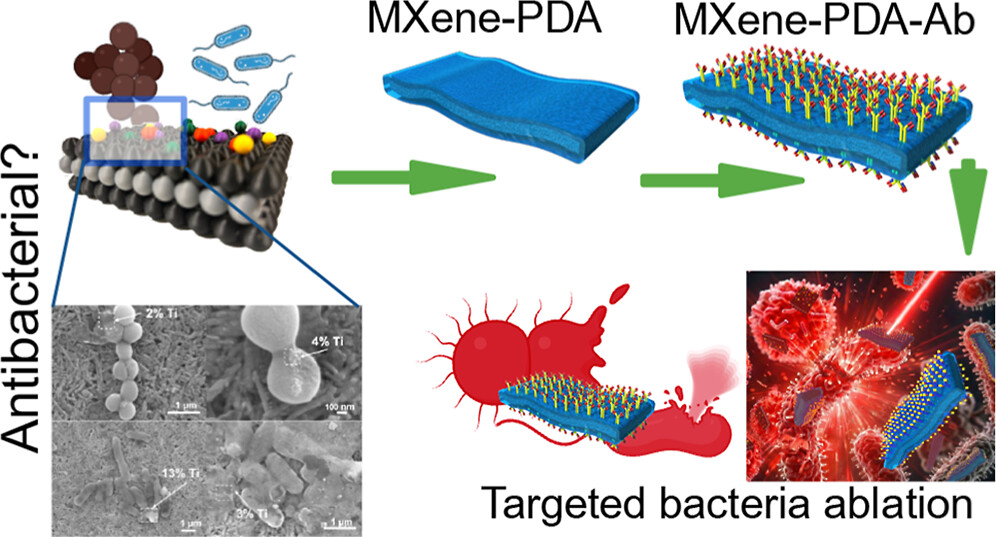 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!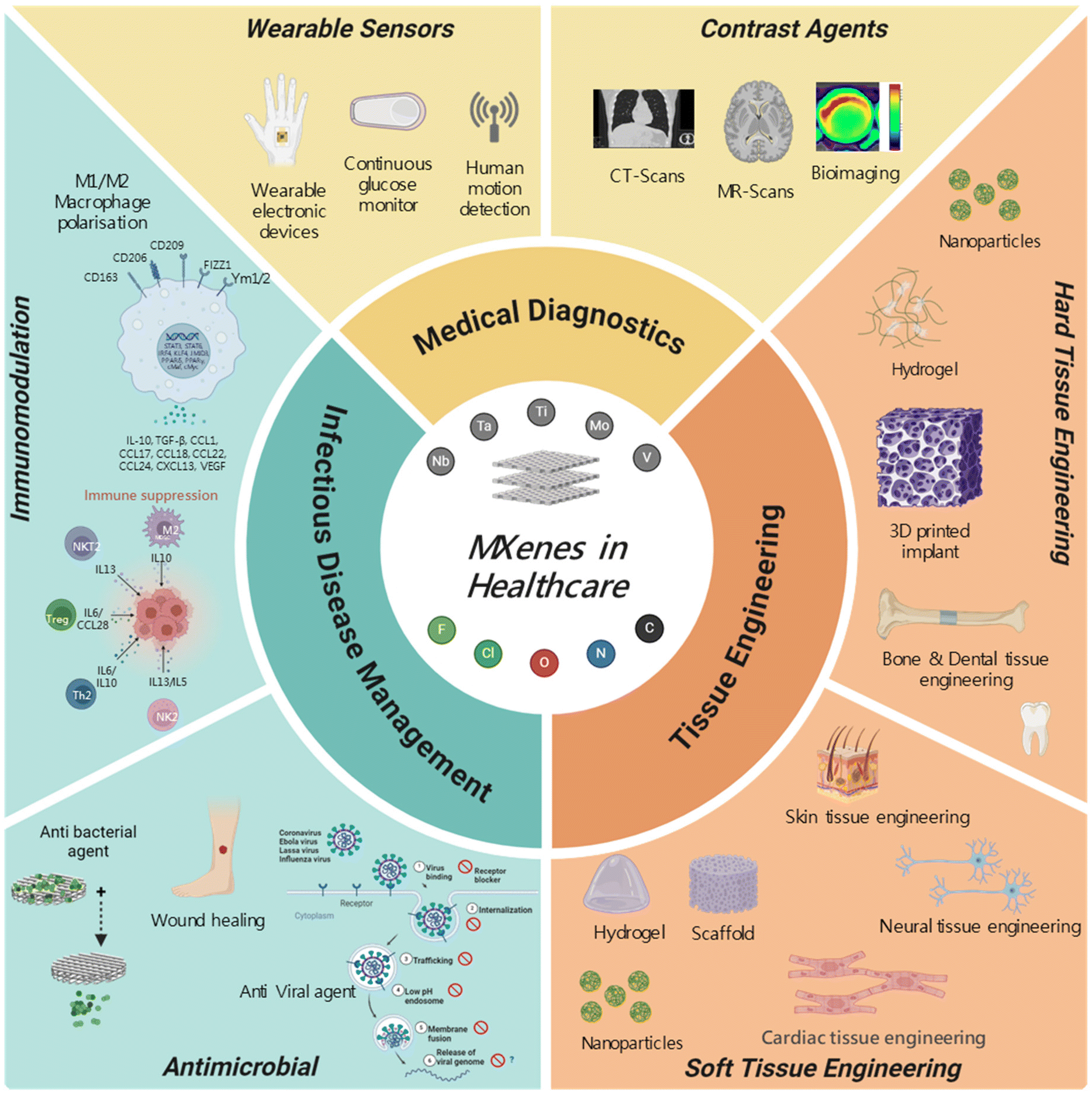 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.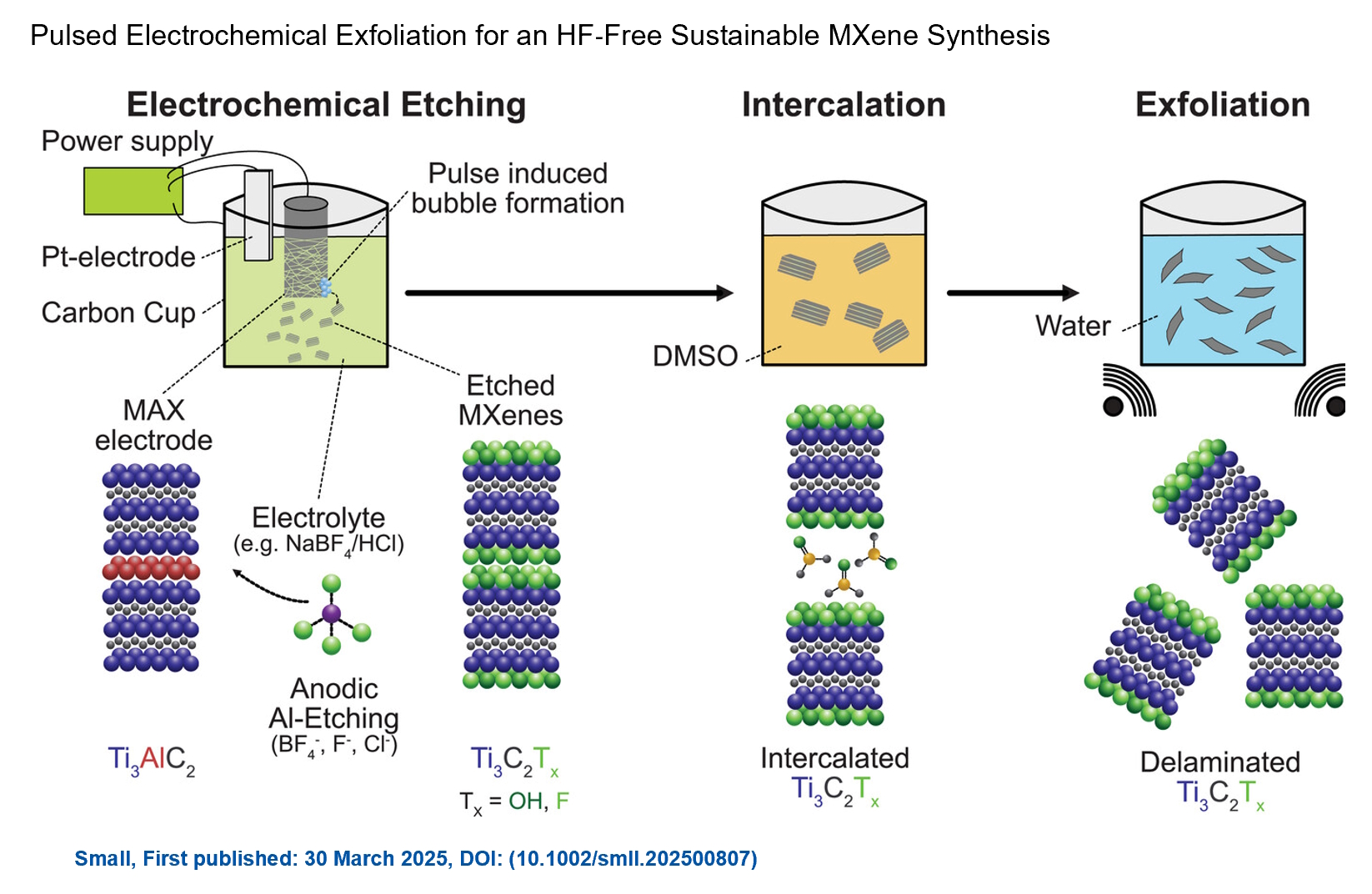 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.