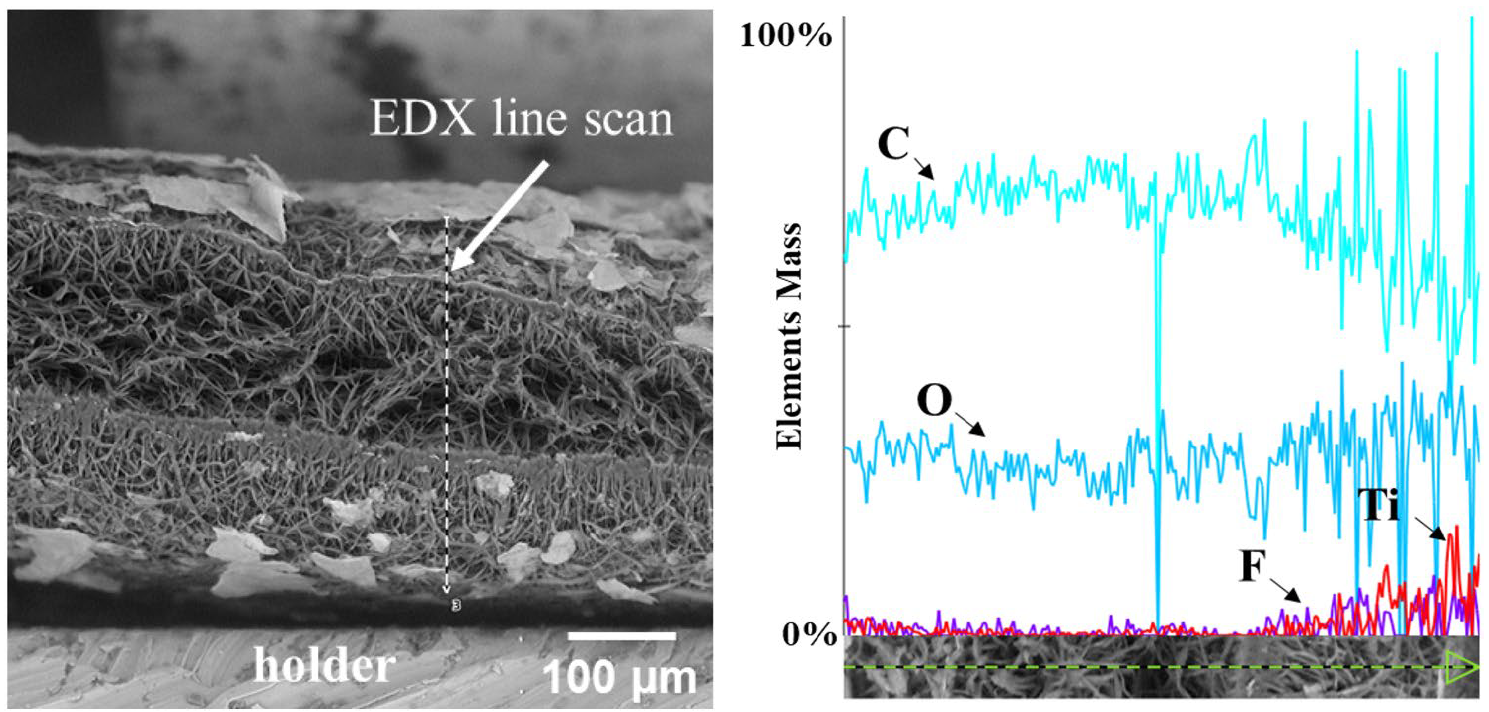
 Here we demonstrate a new developed method for depositing Ti3C2Tx MXenes onto hydrophobic electrospun PCL membranes using oxygen plasma treatment. These novel patches hold tremendous potential for providing mechanical support to damaged heart tissue and enabling electrical signal transmission,thereby mimicking the crucial electroconductivity required for normal cardiac function. After a detailed investigation of scaffold-to-cell interplay, including electrical stimulation, novel technology has the potential for clinical application not only for cardiac regeneration, but also as neural and muscular tissue substitutes.
Here we demonstrate a new developed method for depositing Ti3C2Tx MXenes onto hydrophobic electrospun PCL membranes using oxygen plasma treatment. These novel patches hold tremendous potential for providing mechanical support to damaged heart tissue and enabling electrical signal transmission,thereby mimicking the crucial electroconductivity required for normal cardiac function. After a detailed investigation of scaffold-to-cell interplay, including electrical stimulation, novel technology has the potential for clinical application not only for cardiac regeneration, but also as neural and muscular tissue substitutes.
Novel electrically conductive electrospun PCL‑MXene scaffolds for cardiac tissue regeneration
Kateryna Diedkova1,2 · Yevheniia Husak1,3 · Wojciech Simka3 · Viktoriia Korniienko1,2 · Bojan Petrovic4 · Anton Roshchupkin1 · Agnieszka Stolarczyk3 · Natalia Waloszczyk3 · Ilya Yanko1 · Kaspars Jekabsons2 · Maria Čaplovičová5 · Alexander D. Pogrebnjak1,6 · Veronika Zahorodna7 · Oleksiy Gogotsi7 · Iryna Roslyk7 · Ivan Baginskiy1,7 · Marko Radovic8 · Sanja Kojic9 · Una Riekstina2 · Maksym Pogorielov1,2
1 Sumy State University, 2 Rymskogo‑Korsakova St, Sumy 40007, Ukraine
2 University of Latvia, 3 Jelgavas St, Riga 1004, Latvia
3 Faculty of Chemistry, Silesian University of Technology, 9, Strzody St, 44‑100 Gliwice, Poland
4 Faculty of Medicine, University of Novi Sad, Hajduk Veljkova 3, 21000 Novi Sad, Serbia
5 Centre for Nanodiagnostics of Materials, Slovak, University of Technology in Bratislava, 5 Vazovova St, Bratislava 812 43, Slovakia
6 Faculty of Material Science in Trnava, Institute of Materials, Slovak University of Technology in Bratislava, Jana Bottu c2781/25, Trnava, Slovakia
7 Materials Research Centre, 3 Krzhizhanovskogo St, Kyiv 03142, Ukraine
8 University of Novi Sad, BioSense Institute, Dr Zorana Djindjica 1, 21000 Novi Sad, Serbia
9 Faculty of Technical Sciences, University of Novi Sad, Trg Dositeja Obradovića 6, 21000 Novi Sad, Serbia
Effective cardiac tissue regeneration necessitates scaffolds that mimic the native extracellular matrix and possess desirable properties, such as electrical conductivity and biocompatibility. The choice of an appropriate fabrication method is paramount in achieving reproducibility, scalability, and rapid production of cardiac tissue patches. Electrospinning, a versatile and widely utilized technique, offers precise control over fiber diameter, pore size, and alignment, rendering it an ideal method for creating intricate cardiac scaffolds. In light of the limitations of existing therapies and the need for innovative approaches, this research aims to explore the development of novel patches for cardiac tissue regeneration. By investigating the integration of MXenes into electrospun polycaprolactone (PCL) membranes, we aim to harness the unique properties of MXenes to create conductive, biocompatible, and mechanically robust scaffolds that promote cell adhesion, proliferation, and functional maturation.

The application of oxygen plasma treatment enhances the infiltration of MXene into the PCL electrospun membrane, significantly reducing the surface contact angle and promoting cell adhesion. Regardless of the number of MXene deposition repetitions, all variants demonstrated strong biocompatibility and supported the formation of cell symplasts after fibroblast seeding. The remarkable electrical conductivity of PCL-MXene membranes, coupled with the positive biological outcomes presented in this study, has the potential to drive significant advancements in the field of cardiac tissue engineering. This research offers fresh insights and approaches to tackle the challenges associated with myocardial repair and regeneration.
In this research, we have developed a new method for depositing Ti3C2Tx MXenes onto hydrophobic electrospun PCL membranes using oxygen plasma treatment. This innovative approach has demonstrated a positive impact on fiber size, increased the porous structure and significantly reducing the contact angle of the PCL membrane and facilitating deep impregnation of MXene into the material. The resulting PCL-MXene composite membrane is non toxic, exhibits the desired conductive properties necessary for cardiac tissue regeneration, with no significant differences observed between the various numbers of MXene depositions. Overall, the incorporation of MXenes into biodegradable PCL membranes shows promise in conferring electroconductivity and enhancing cellular response in tissue-engineered cardiac patches.
These novel patches hold tremendous potential for providing mechanical support to damaged heart tissue and enabling electrical signal transmission,thereby mimicking the crucial electroconductivity required for normal cardiac function. After a detailed investigation of scaffold-to-cell interplay, including electrical stimulation, novel technology has the potential for clinical application not only for cardiac regeneration, but also as neural and muscular tissue substitutes.
Keywords: MXene · PCL · Electroconductive electrospun membrane · Oxygen plasma treatment · Tissue regeneration
Read more on the publisher`s website:
Diedkova, K., Husak, Y., Simka, W. et al. Novel electrically conductive electrospun PCL-MXene scaffolds for cardiac tissue regeneration. Graphene and 2D Mater (2023). https://doi.org/10.1007/s41127-023-00071-5
MXene-Assisted Ablation of Cells with a Pulsed Near-Infrared Laser
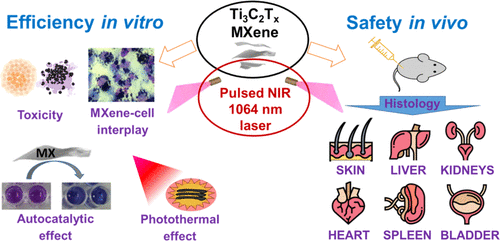 Presenting our recent collaborative research paper on MXene use for PPT anticancer therapy, the biocompatibility of MXenes in vitro and in vivo studies:
Presenting our recent collaborative research paper on MXene use for PPT anticancer therapy, the biocompatibility of MXenes in vitro and in vivo studies:
Sergiy Kyrylenko, Oleksiy Gogotsi, Ivan Baginskiy, Vitalii Balitskyi, Veronika Zahorodna, Yevheniia Husak, Ilya Yanko, Mykolay Pernakov, Anton Roshchupkin, Mykola Lyndin, Bernhard B. Singer, Volodymyr Buranych, Alexander Pogrebnjak, Oksana Sulaieva, Oleksandr Solodovnyk, Yury Gogotsi, Maksym Pogorielov, MXene-Assisted Ablation of Cells with a Pulsed Near-Infrared Laser. ACS Appl. Mater. Interfaces 2022, 14, 25, 28683–28696, https://doi.org/10.1021/acsami.2c08678



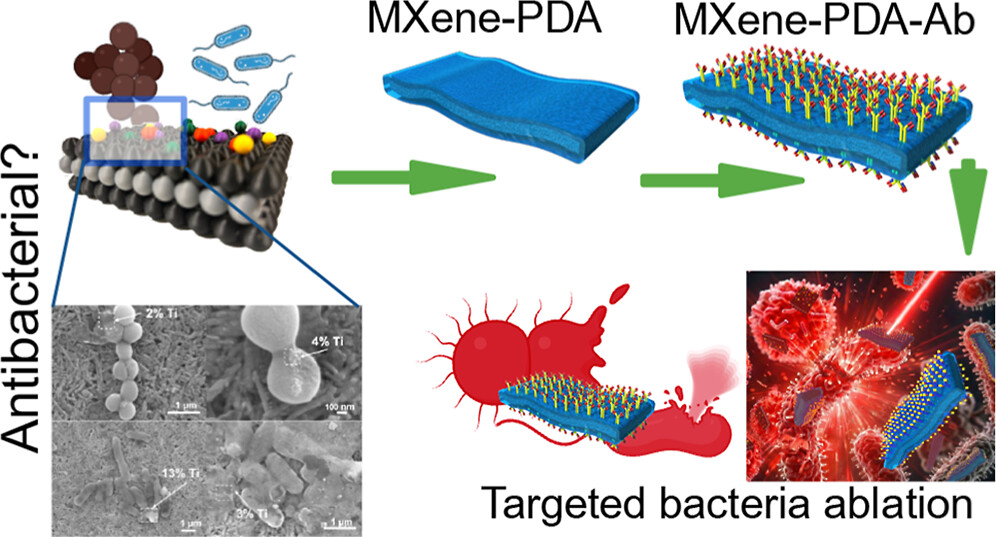 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
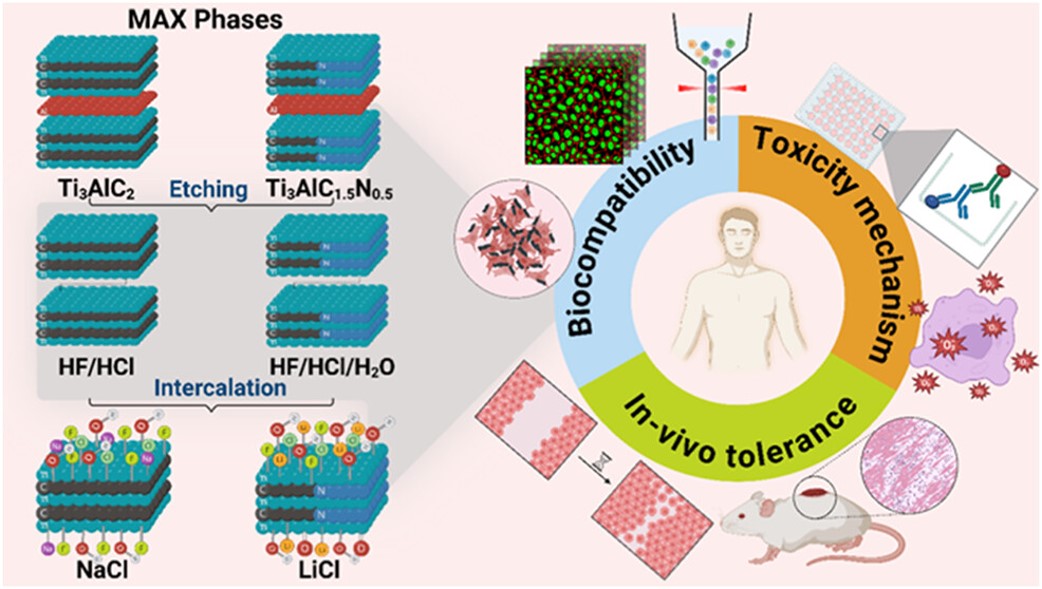 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.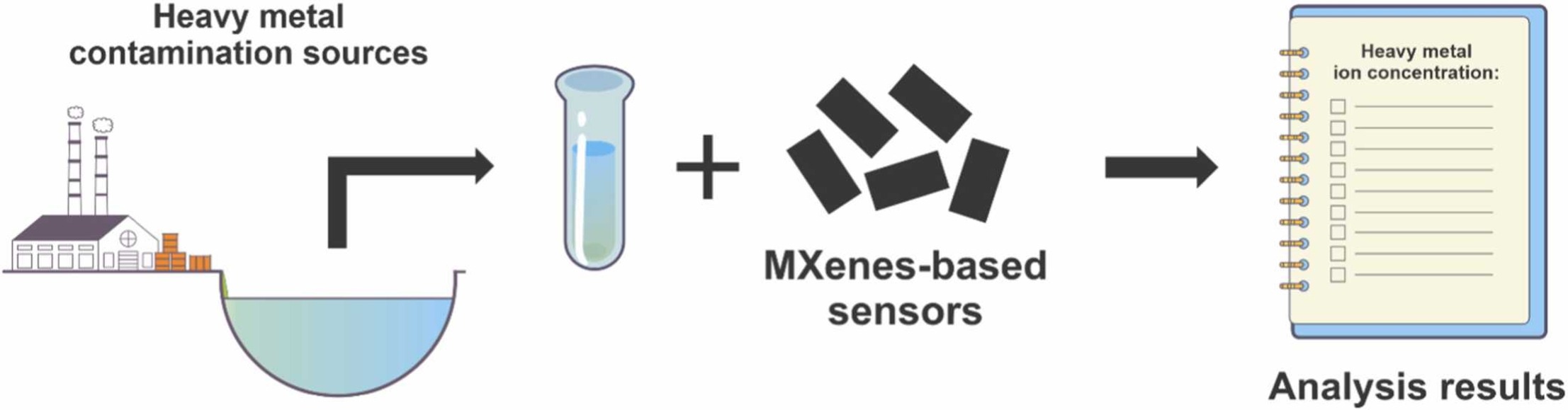 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!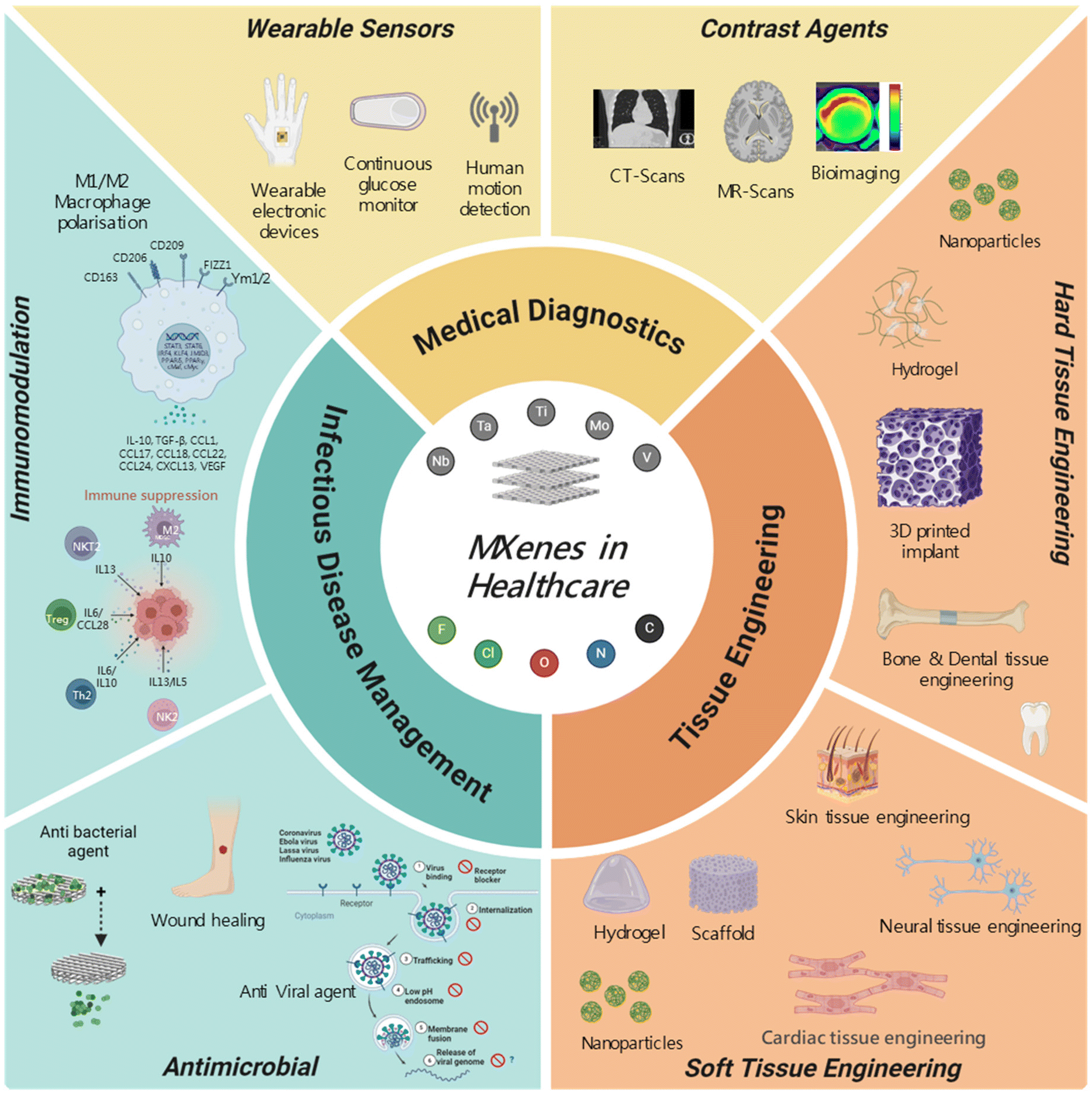 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.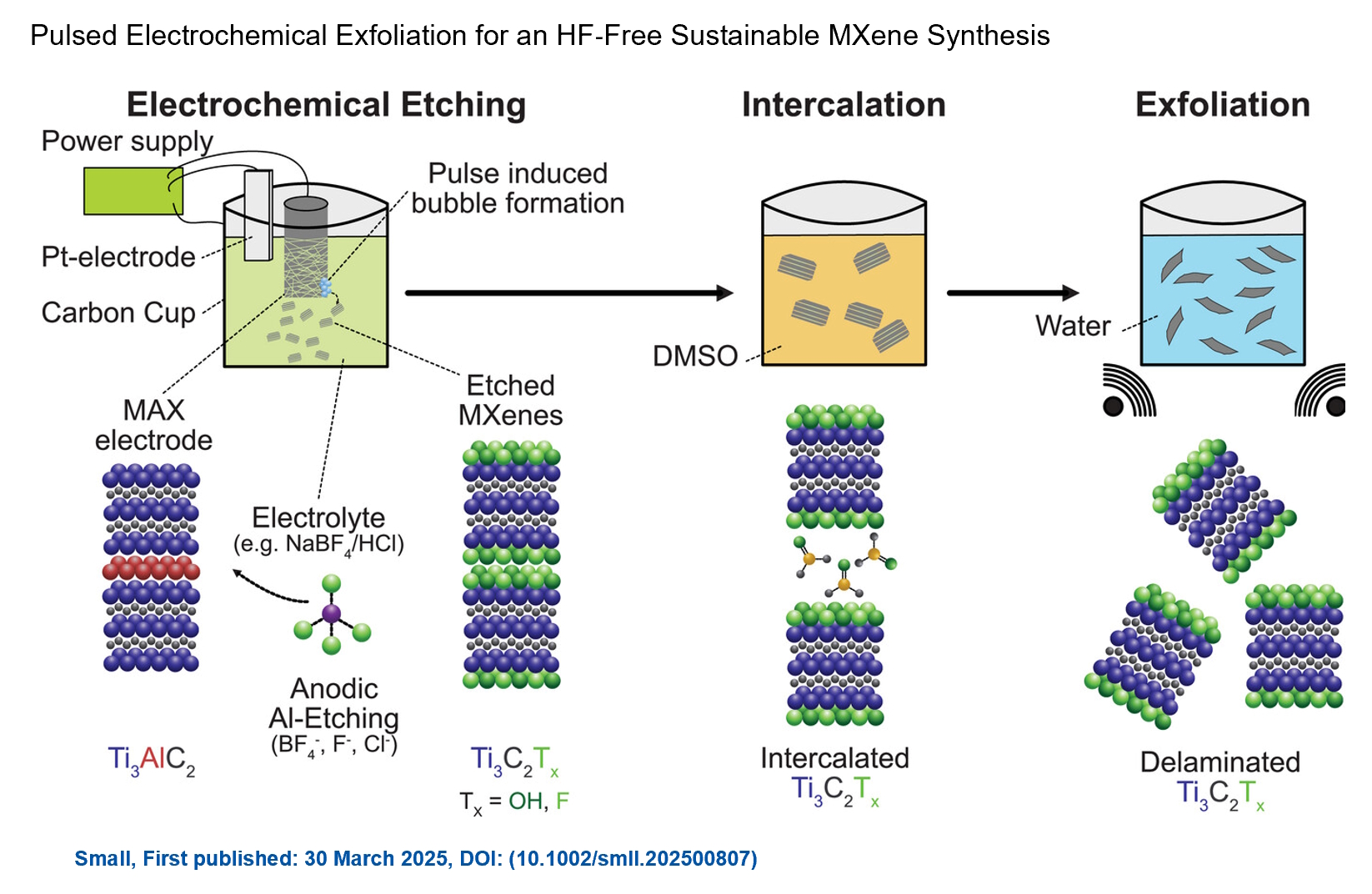 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.