Photothermal therapy (PTT) is a method for eradicating tumor tissues through the use of photothermal materials and photosensitizing agents that absorb light energy from laser sources and convert it into heat, which selectively targets and destroys cancer cells while sparing healthy tissue.
MXenes have been intensively investigated as photosensitizing agents for PTT. However, achieving the selectivity of MXenes to the tumor cells remains a challenge. Specific antibodies (Ab) against tumor antigens can achieve homing of the photosensitizing agents toward tumor cells, but their immobilization on MXene received little attention. Here, we offer a strategy for the selective ablation of melanoma cells using MXene-polydopamineantiCEACAM1 Ab complexes. We coated Ti3C2Tx MXene with polydopamine (PDA), a natural compound that attaches Ab to the MXene surface, followed by conjugation with an anti-CEACAM1 Ab. Our experiments confirm the biocompatibility of the Ti3C2Tx-PDA and Ti3C2Tx-PDAantiCEACAM1 Ab complexes across various cell types. We also established a protocol for the selective ablation of CEACAM1- positive melanoma cells using near-infrared irradiation. The obtained complexes exhibit high selectivity and efficiency in targeting and eliminating CEACAM1-positive melanoma cells while sparing CEACAM1-negative cells. These results demonstrate the potential of MXene-PDA-Ab complexes for cancer therapy. They underline the critical role of targeted therapies in oncology, offering a promising avenue for the precise and safe treatment of melanoma and possibly other cancers characterized by specific biomarkers. Future research will aim to refine these complexes for clinical use, paving the way for new strategies for cancer treatment.
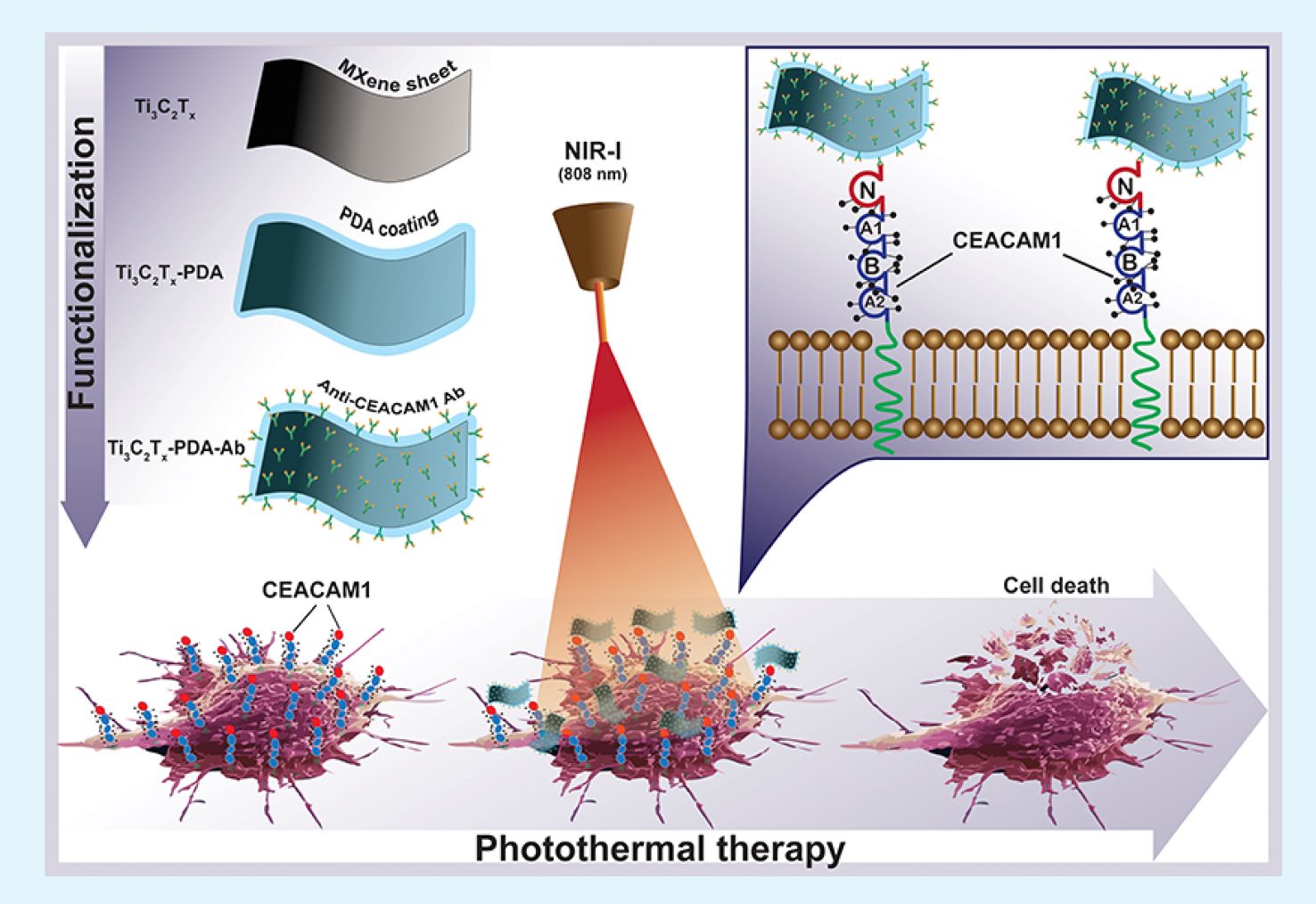
Our study has showcased the potential of the MXene-PDAantiCEACAM1 Ab complex as a highly selective and efficient means for the targeted ablation of melanoma cells. We have introduced a cost-effective, simple, and reproducible technology for modifying Ti3C2Tx MXene using PDA, followed by a one-step mAb binding. This innovative approach represents a significant advance over previous methodologies by combining the high photothermal efficiency of MXenes with the precise targeting capability of antibodies, thereby achieving substantial cell death in CEACAM1-positive melanoma cells with a minimal impact on CEACAM1-negative cells. This strategy holds promise for mitigating the risk of damage to surrounding healthy tissues, a prevalent issue in traditional cancer therapies.
The efficacy of these complexes in selectively binding and ablating targeted cells underscores the critical role of combining nanomaterials with specific biological markers in developing targeted cancer therapies.
KEYWORDS: MXene, photothermal ablation, targeted delivery, CEACAM1, antibody immobilization, anticancer therapy
To cite: Anastasia Konieva, Volodymyr Deineka, Kateryna Diedkova, Daniel Aguilar-Ferrer, Mykola Lyndin, Gunther Wennemuth, Viktoriia Korniienko, Sergiy Kyrylenko, Alexey Lihachev, Veronika Zahorodna, Ivan Baginskiy, Emerson Coy, Oleksiy Gogotsi, Agata Blacha-Grzechnik, Wojciech Simka, Irina Kube-Golovin, Igor Iatsunskyi, and Maksym Pogorielov. MXene-Polydopamine-antiCEACAM1 Antibody Complex as a Strategy for Targeted Ablation of Melanoma. ACS Applied Materials & Interfaces 2024 16 (33), 43302-43316 DOI: 10.1021/acsami.4c08129
Read more about this work in ACS Appl. Mater. Interfaces: https://pubs.acs.org/doi/full/10.1021/acsami.4c08129
Other recent papers:
| Novel electrically conductive electrospun PCL‑MXene scaffolds for cardiac tissue regeneration |
|
|
| MXene Functionalized Kevlar Yarn via Automated, Continuous Dip Coating |
|
|


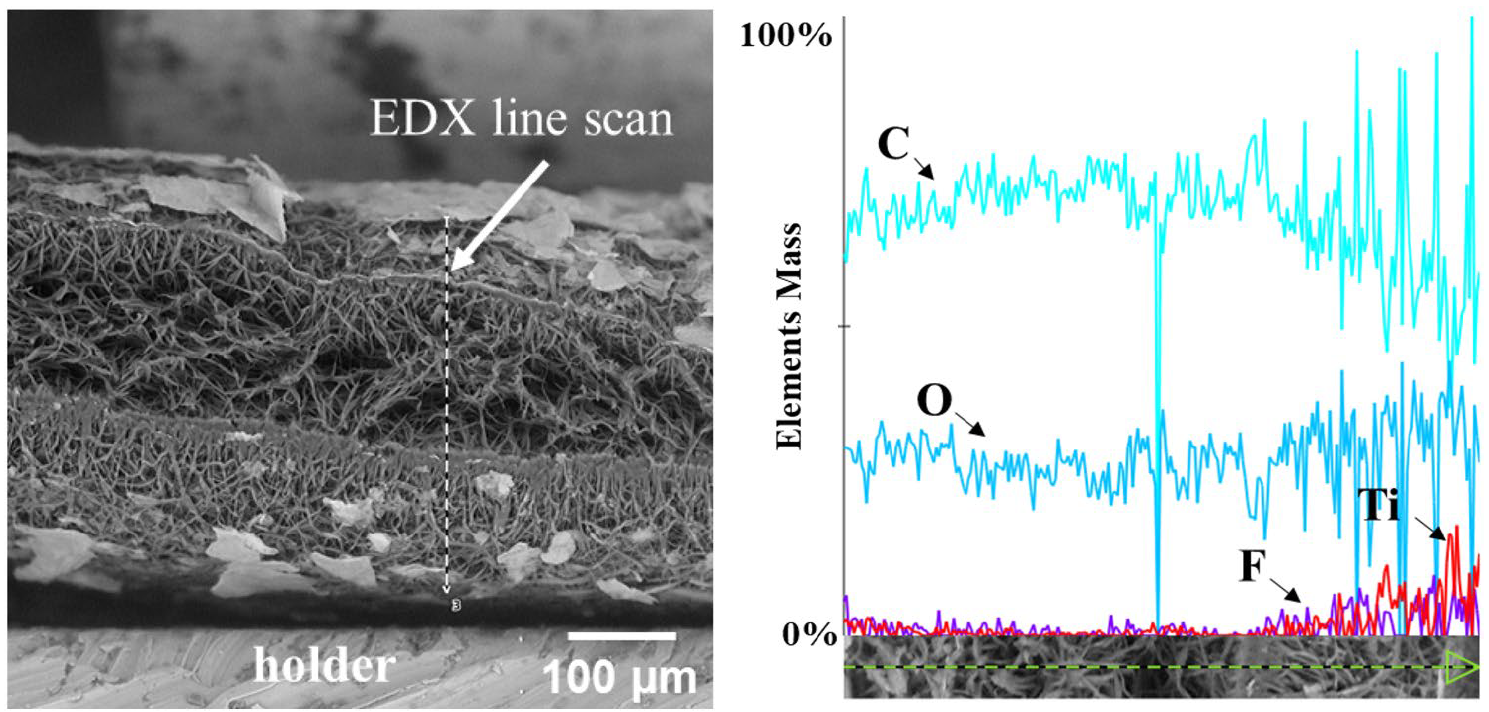 Here we demonstrate a new developed method for depositing Ti3C2Tx MXenes onto hydrophobic electrospun PCL membranes using oxygen plasma treatment. These novel patches hold tremendous potential for providing mechanical support to damaged heart tissue and enabling electrical signal transmission,thereby mimicking the crucial electroconductivity required for normal cardiac function.
Here we demonstrate a new developed method for depositing Ti3C2Tx MXenes onto hydrophobic electrospun PCL membranes using oxygen plasma treatment. These novel patches hold tremendous potential for providing mechanical support to damaged heart tissue and enabling electrical signal transmission,thereby mimicking the crucial electroconductivity required for normal cardiac function. 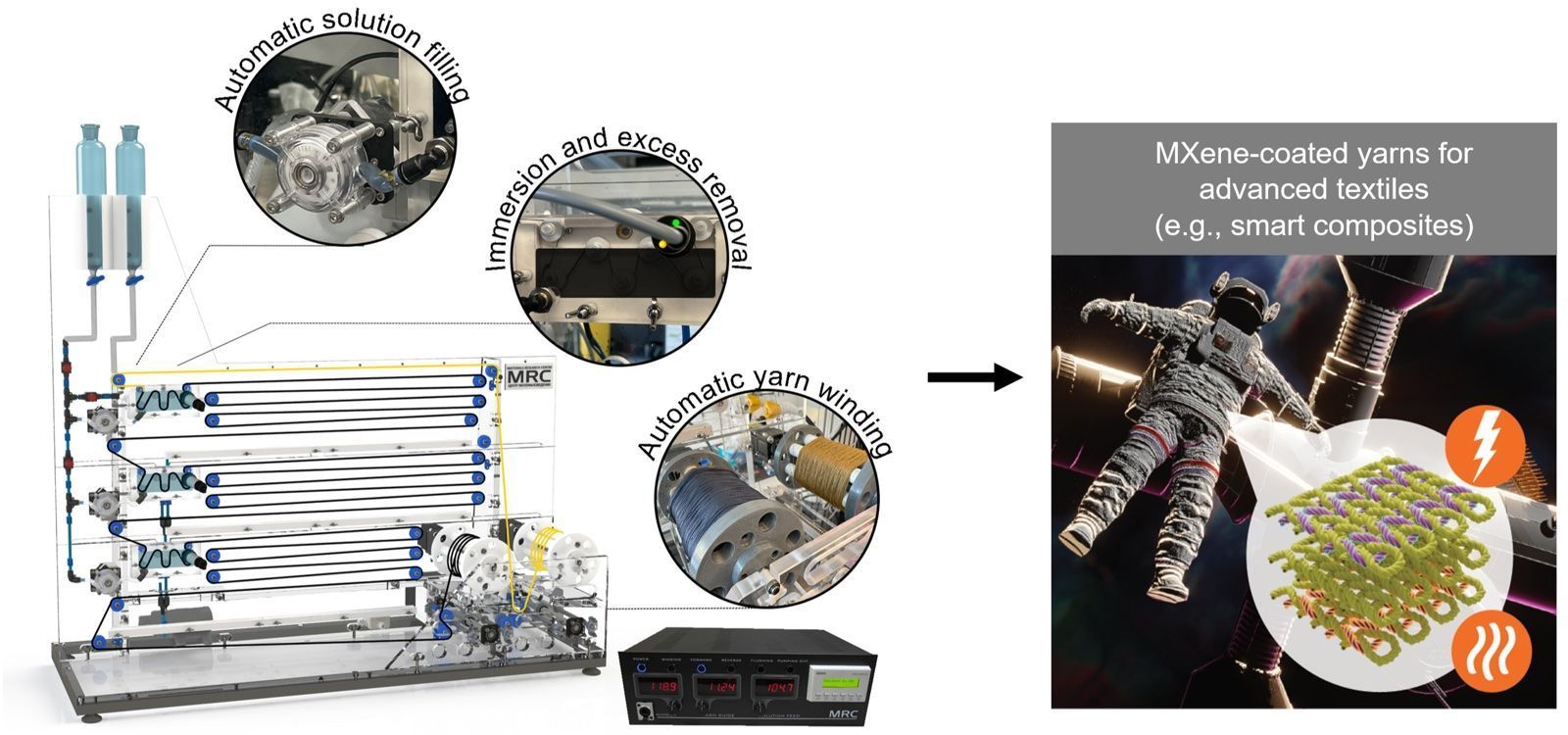 The rise of the Internet of Things has spurred extensive research on integrating conductive materials into textiles to turn them into sensors, antennas, energy storage devices, and heaters. MXenes, owing to their high electrical conductivity and solution processability, offer an efficient way to add conductivity and electronic functions to textiles. Here, a versatile automated yarn dip coater tailored for producing continuously high-quality MXene-coated yarns and conducted the most comprehensive MXene-yarn dip coating study to date is developed.
The rise of the Internet of Things has spurred extensive research on integrating conductive materials into textiles to turn them into sensors, antennas, energy storage devices, and heaters. MXenes, owing to their high electrical conductivity and solution processability, offer an efficient way to add conductivity and electronic functions to textiles. Here, a versatile automated yarn dip coater tailored for producing continuously high-quality MXene-coated yarns and conducted the most comprehensive MXene-yarn dip coating study to date is developed. 
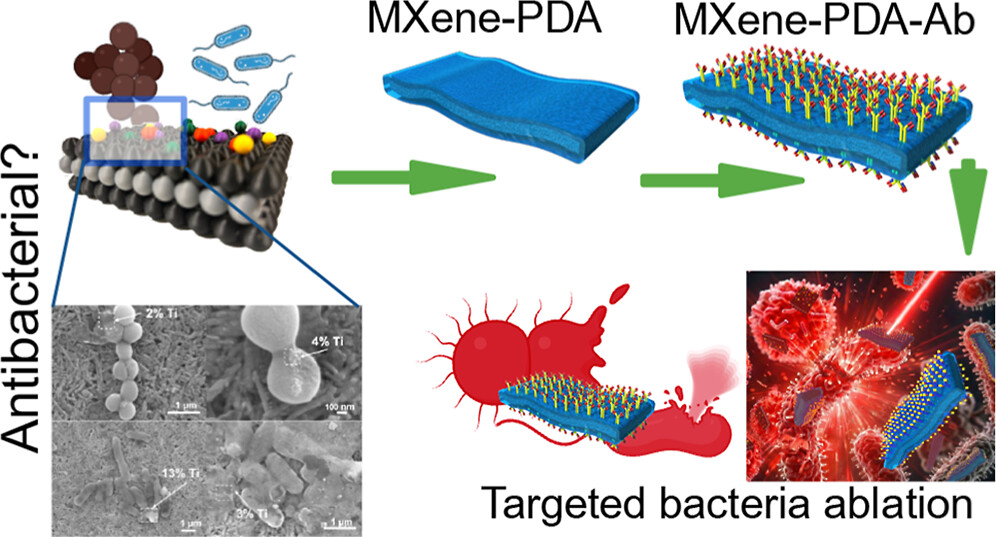 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.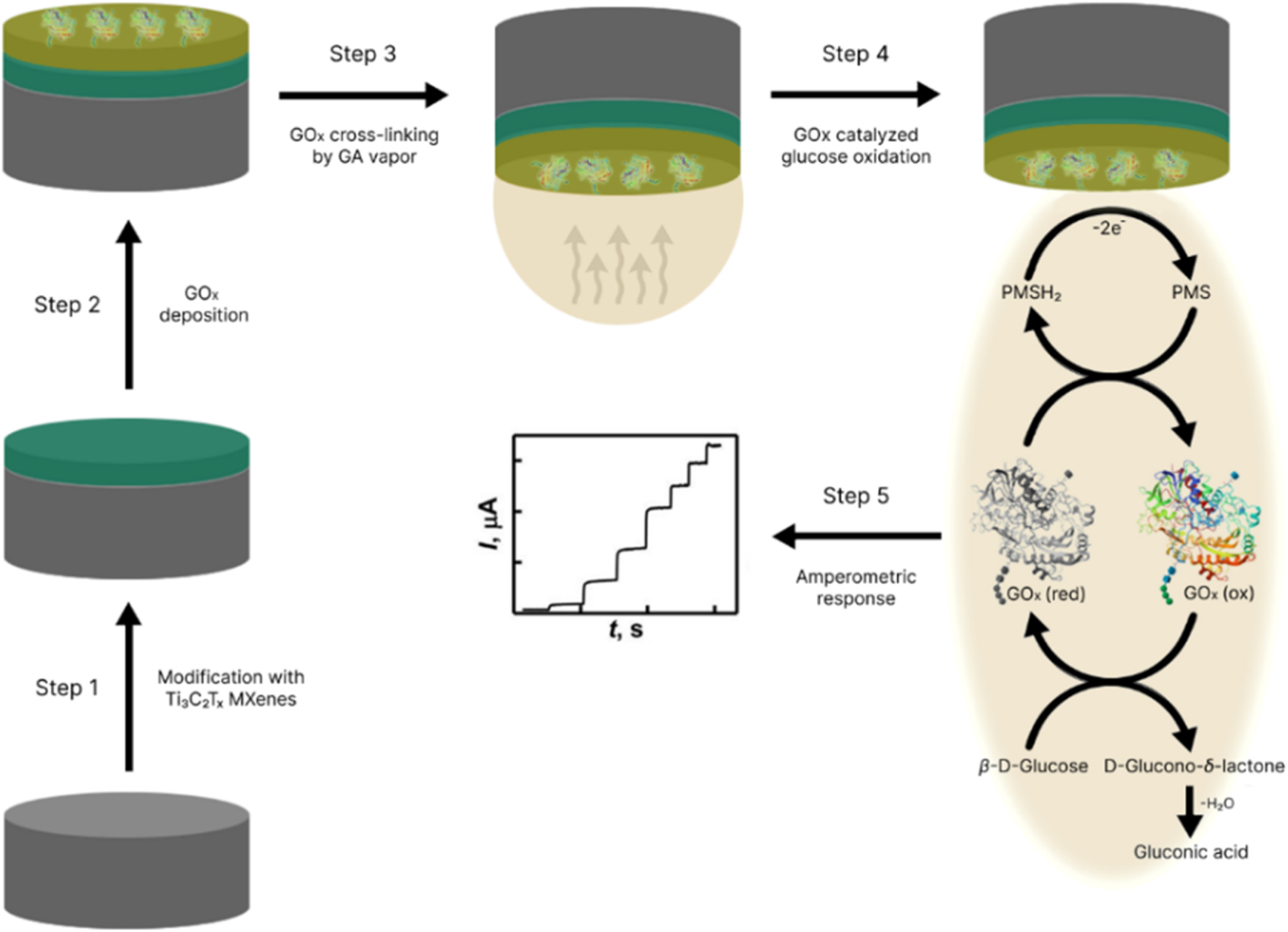
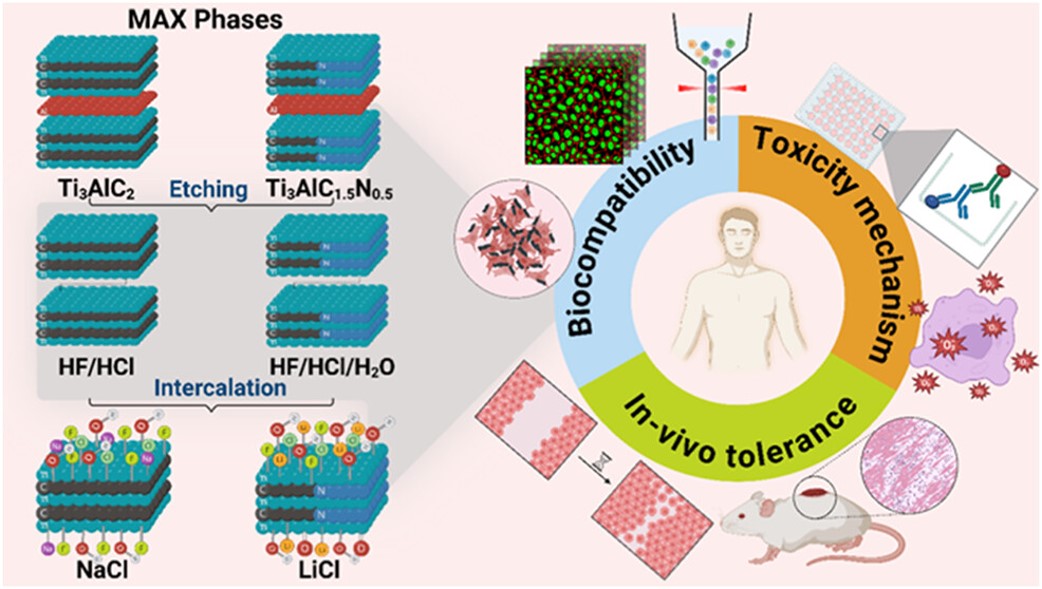 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.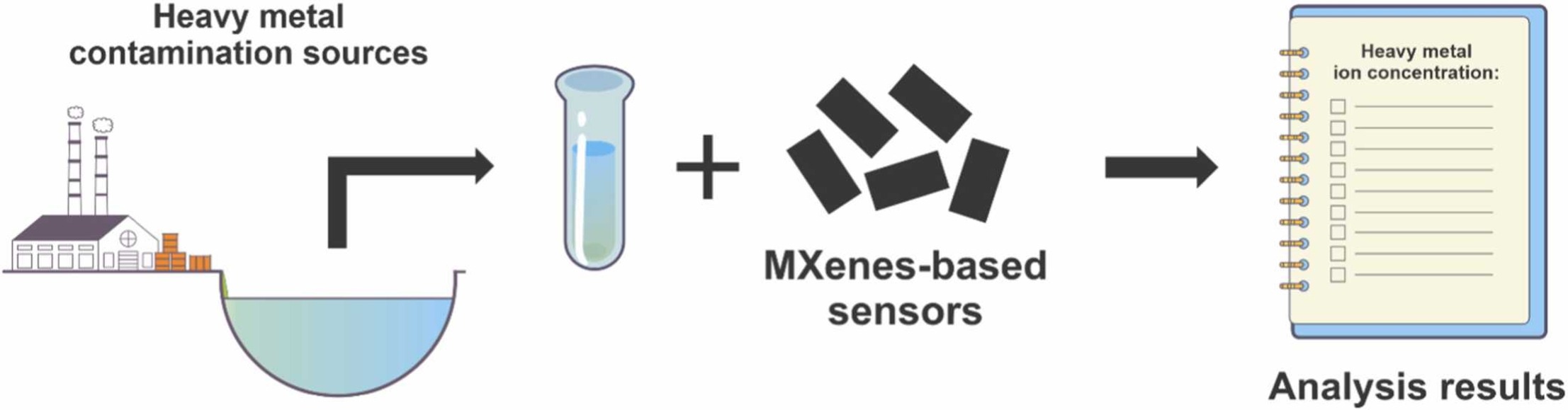 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!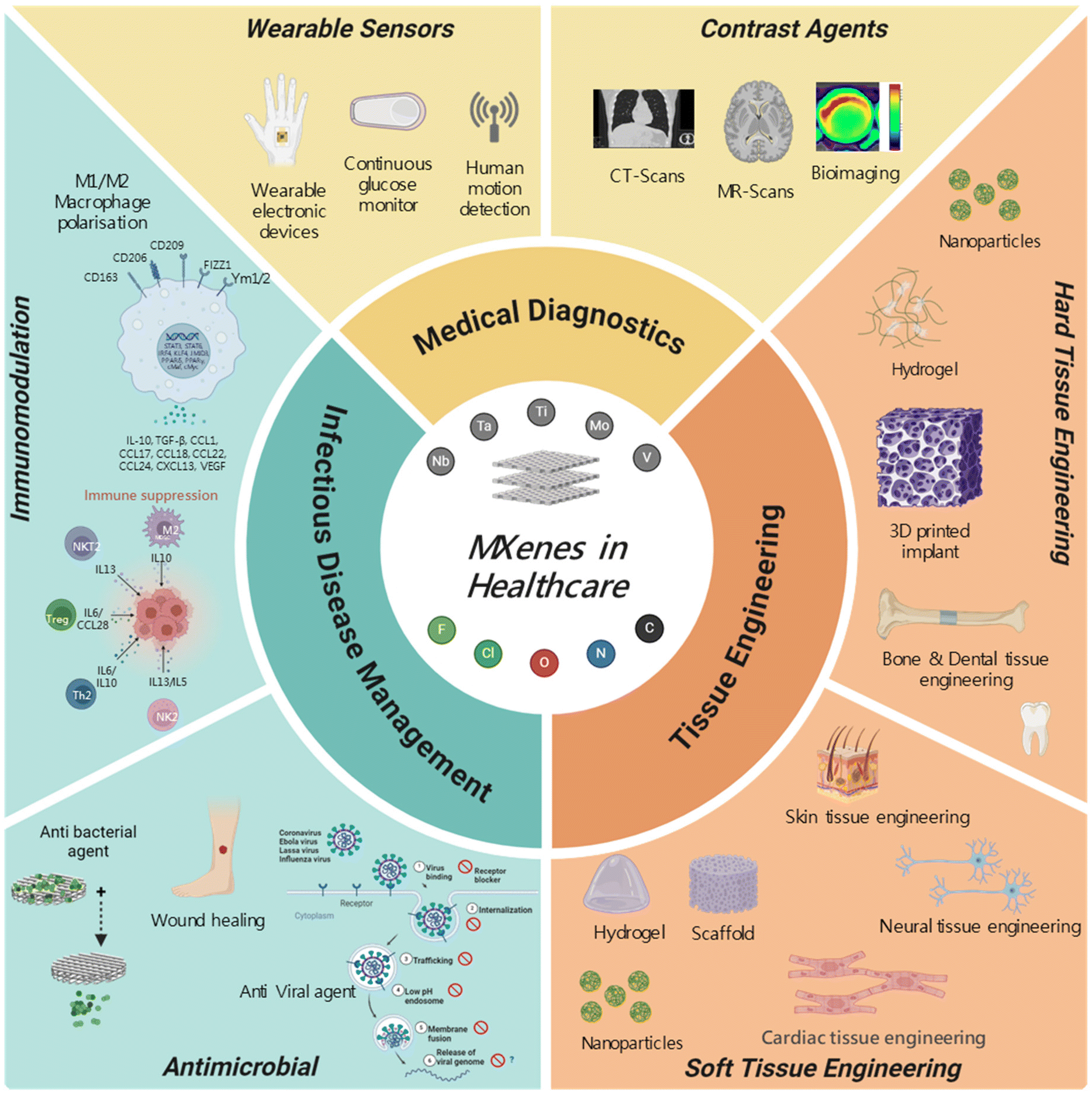 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.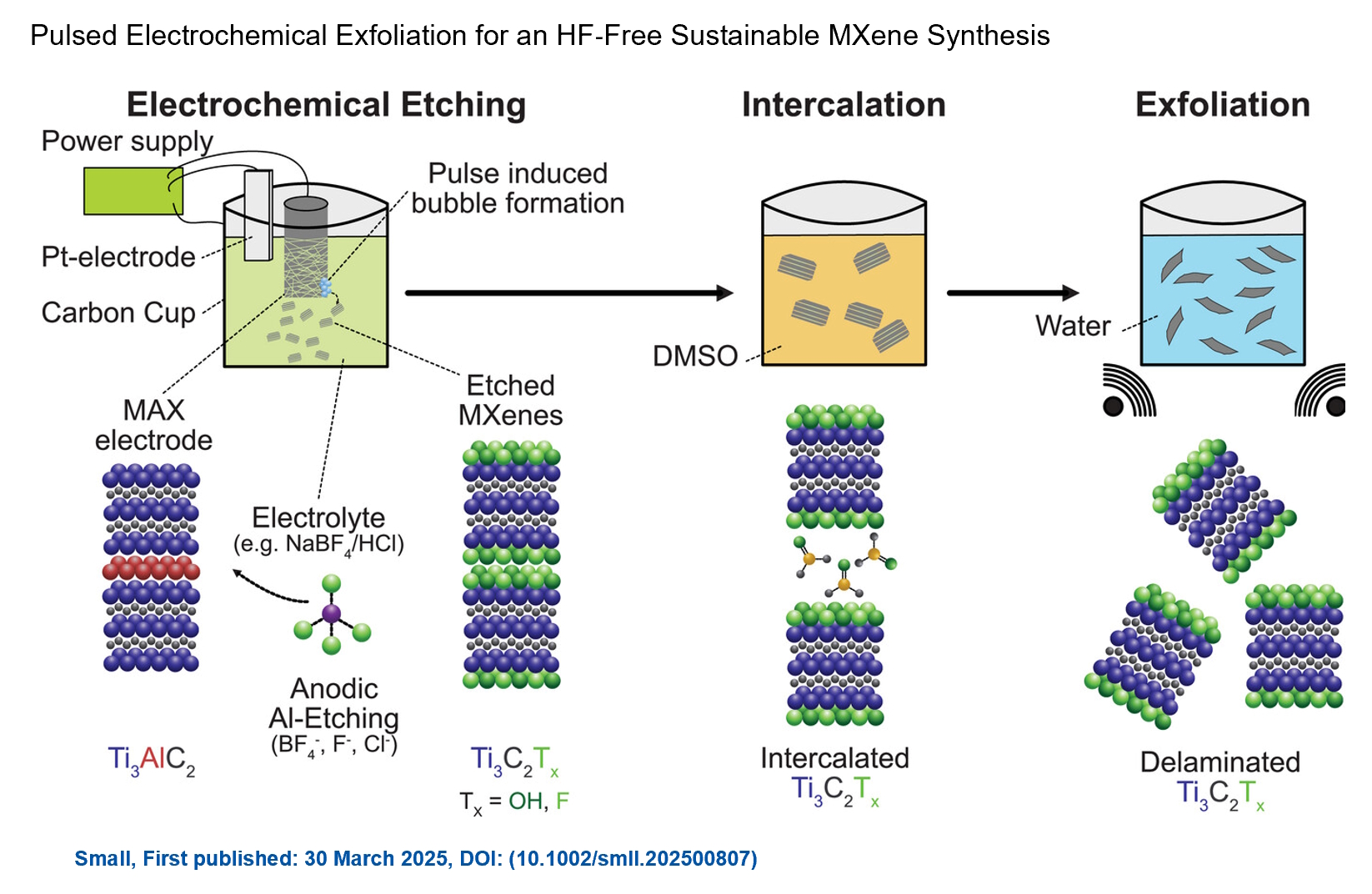 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.