Just Squeeze In — Drexel Researchers Discover When Spaces Are Tight, Nature Loosens Its Laws
It turns out that when they’re in a hurry and space is limited, ions, like people, will find a way to cram in — even if that means defying nature’s norms. Recently published research from an international team of scientists, including Drexel University’s Yury Gogotsi, PhD, shows that the charged particles will actually forgo their “opposites attract” behavior, called Coulombic ordering, when confined in the tiny pores of a nanomaterial. This discovery could be a pivotal development for energy storage, water treatment and alternative energy production technologies, which all involve ions packing into nanoporous materials.
In their paper, which was recently published in the journal Nature Materials, the researchers explain how Coulombic ordering in liquid salts starts to break down when ions are confined in small spaces — specifically carbon pores less than a nanometer in diameter. And the narrower the pore, the less the ions adhere to Coulombic ordering.
“This is the first time breaking of the Coulombic ordering in subnanometer pores has been convincingly demonstrated,” said Gogotsi, an author of the paper, who is the Distinguished University and Bach professor in Drexel’s College of Engineering. “The breaking of symmetry principals, like Coulombic ordering, plays an essential role in nature. But many of these processes occur without us understanding them and knowing their mechanisms. Science can reveal those hidden processes. And if we understand them, we can eventually develop better technology by working at the same nanometer and subnanometer scales that nature does.”
To make its discovery, the team — including researchers from Shinshu University in Japan; Loughborough University in the United Kingdom; The University of Adelaide in Australia; and Sorbonne University, the French Research Network on Electrochemical Energy Storage, and Paul Sabatier University in France — created two sets of carbon nanomaterials. One had pores at least a nanometer in diameter and one with pores less than a nanometer. They then used the materials to draw in ionic liquid as if they were a sponge sopping up water.
In ionic liquids, which are room-temperature liquid salts often used as solvents in the chemical industry, ions are layered in full compliance with the alternating positive-negative pattern of Coulombic ordering. But as the ionic liquid drew into the carbon nanopores it forced the ions to line up in single- and double-file lines. And, like a flock of elementary schoolers running for the bus, they didn’t always end up in line next to their usual cohorts.
“In this state, the Coulombic ordering of the liquid is broken,” the authors wrote. “Ions of the same charge neighbor each other due to a screening of their electrostatic interactions by the image charges induced in the pore walls.”

The team observed this disruption in the natural order of ions through x-ray scattering and modeled the process to explain the experimental observations. They also reported that the non-Coulombic ordering became more pronounced when an electric charge was applied to the carbon material.
“Our results suggest the existence of a molecular-scale mechanism that reduces the Coulombic repulsion energy between co-ions that become closer to each other,” they wrote. This mechanism, they theorize, is linked to the charge temporarily imposed on the walls of the carbon pores. This “image charge,” they write, offsets the natural electrostatic repulsion of ions of the same charge, to allow the channels to fill with same-charged ions lined up next to each other.
Gogotsi suggests this discovery could make it more feasible to use ionic liquids in batteries and other energy storage devices, which has been examined as a method for making batteries safer but has yet to catch on because it limits their performance.
“We can get safer batteries and supercapacitors when using ionic liquid electrolytes because they are not flammable like the electrolyte solution currently used in these devices,” Gogotsi said. “Also, since there is no solvent, the entire volume is occupied by ions and we may be able to store more energy compared to conventional electrolytes that use organic solvents.”
He’s also looking at this discovery as one that could have a significant impact on the push for water desalination technology. Membranes currently being developed to turn salt water into drinking water could be improved with this knowledge about ion behavior within subnanometer pores.
“This work adds fundamental understanding to how different things can behave below one nanometer in scale,” Gogotsi said. “We go beyond nanotechnology and into sub-nanoscale process — that’s the next frontier.”
Source: http://drexel.edu/now/archive/2017/September/nanopore-non-Coulombic-ordering/




 Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.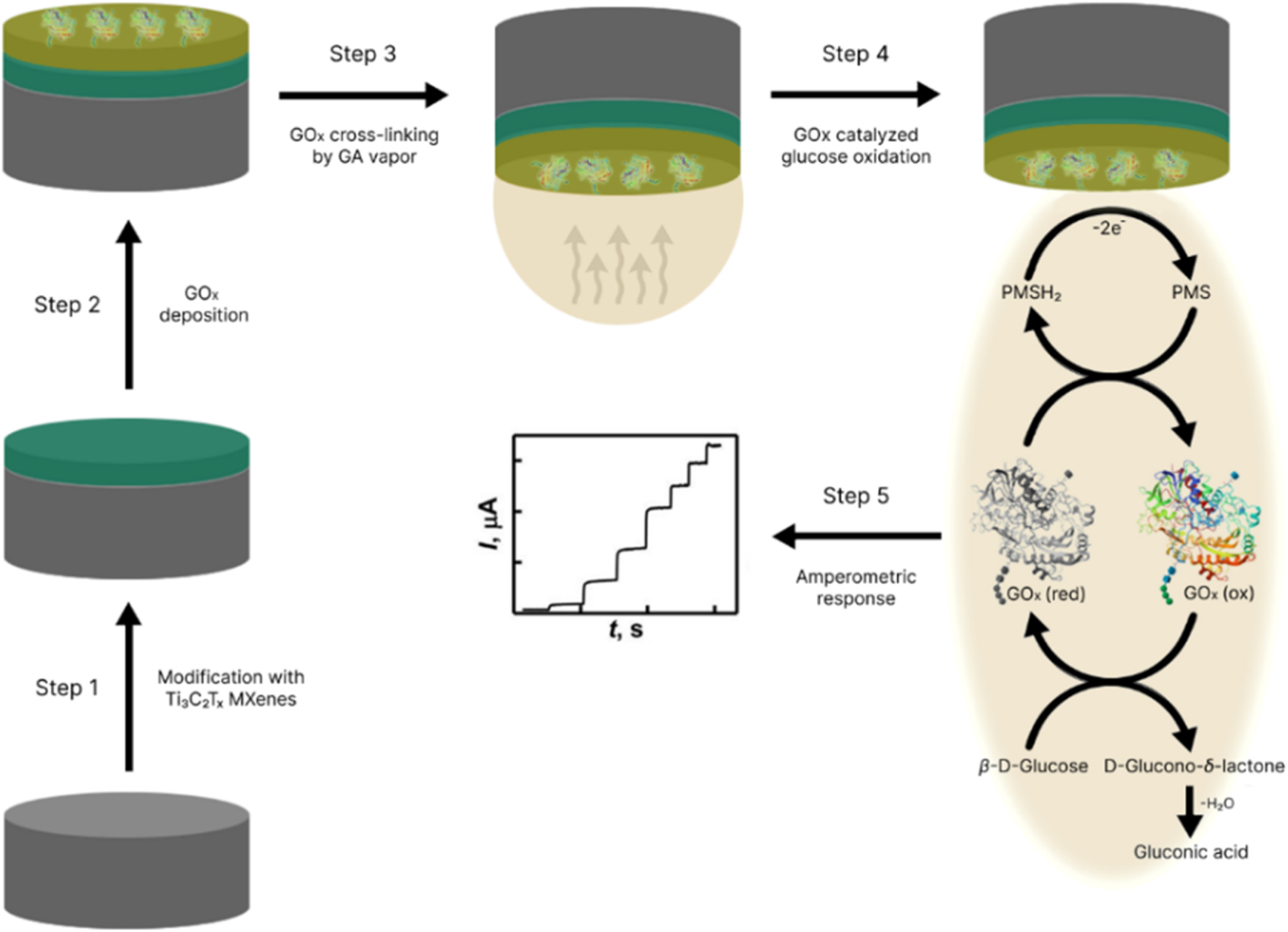
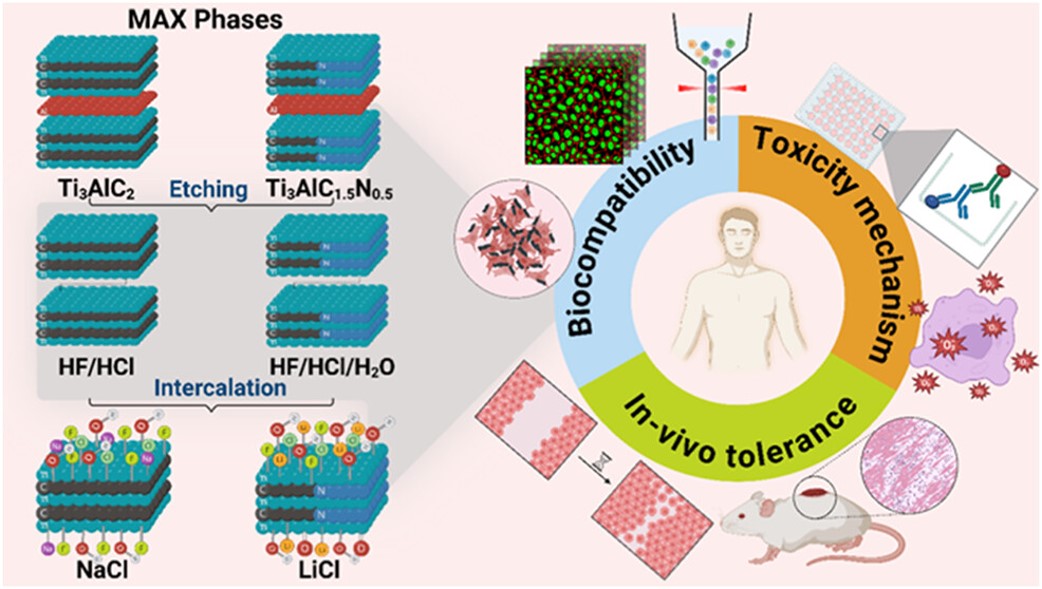 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.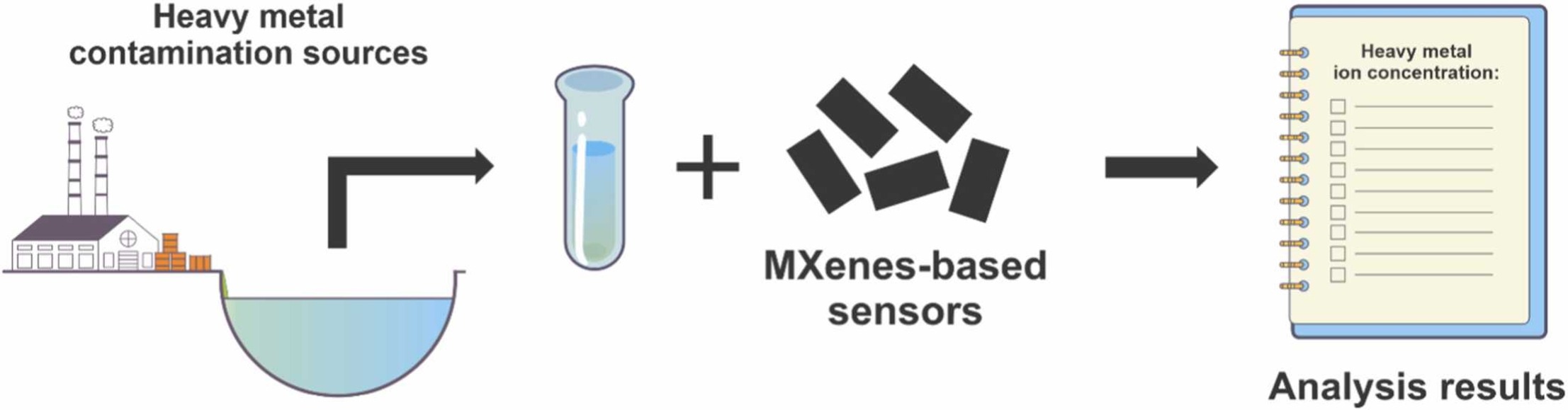 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!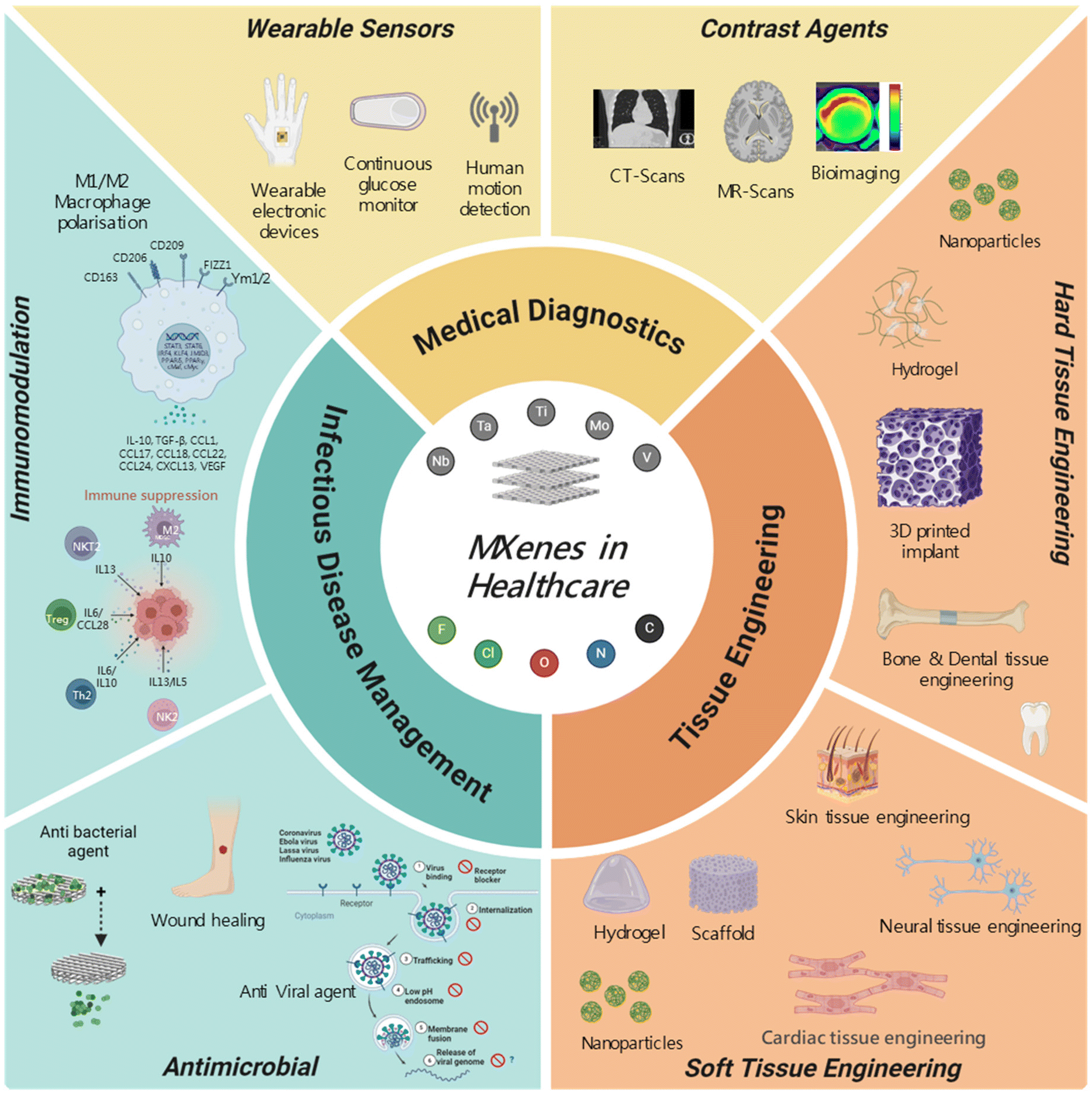 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.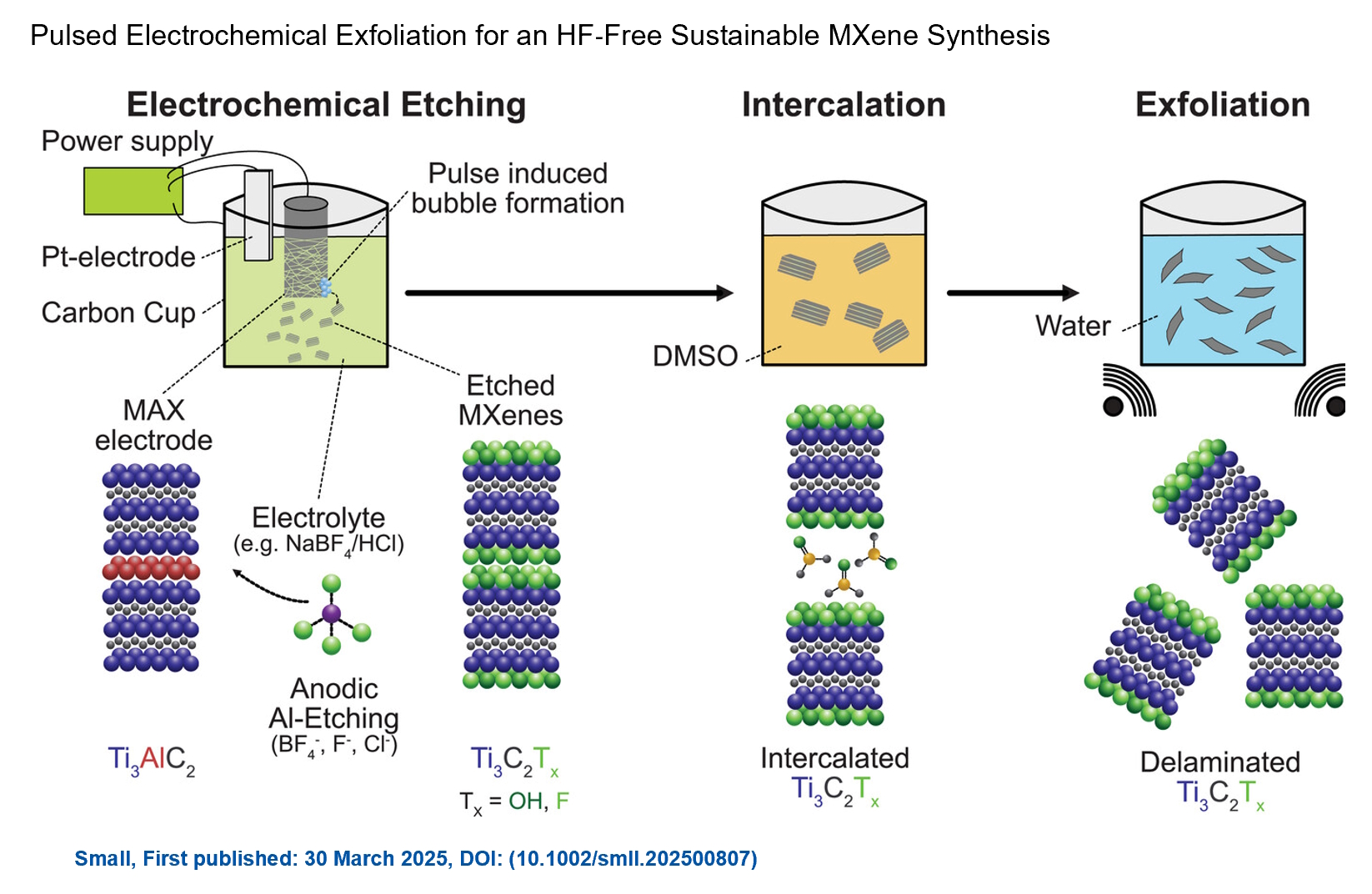 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved. Congratulations to all collaborators with this interesting joint work!
Congratulations to all collaborators with this interesting joint work!