| Rus | Eng |
Strain-Based In Situ Study of Anion and Cation Insertion into Porous Carbon Electrodes with Different Pore Sizes
 Jennifer M. Black1,Guang Feng2,*,Pasquale F. Fulvio3,Patrick C. Hillesheim3,Sheng Dai3,4,Yury Gogotsi5,Peter T. Cummings2,Sergei V. Kalinin1,Nina Balke1,*
Jennifer M. Black1,Guang Feng2,*,Pasquale F. Fulvio3,Patrick C. Hillesheim3,Sheng Dai3,4,Yury Gogotsi5,Peter T. Cummings2,Sergei V. Kalinin1,Nina Balke1,*
1 Center for Nanophase Materials Sciences, Oak Ridge National Laboratory, Oak Ridge, TN, USA
2 Chemical & Biomolecular Engineering, Vanderbilt University, Nashville, TN, USA
3 Chemical Sciences Division Oak Ridge National Laboratory, Oak Ridge, TN, USA
4 Department of Chemistry University of Tennessee, Knoxville, TN, USA
5 Department of Materials Science and Engineering and A. J. Drexel Nanotechnology Institute Drexel University, Philadelphia, PA, USA
Article first published online: 8 OCT 2013, Adv. Energy Mater.,DOI: 10.1002/aenm.201300683
Keywords: electrochemical capacitors; atomic force microscopy; molecular dynamics; ionic liquids
Abstract
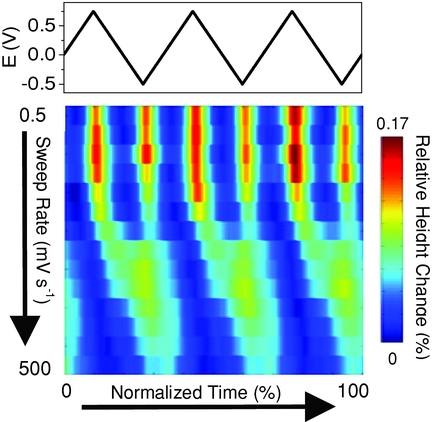 Atomic force microscopy is used to monitor the expansion of porous carbon electrodes, which results from insertion/adsorption of ions in carbon pores during charging. The strain data collected at various potential scan rates are used to obtain information on anion and cation kinetics. Molecular dynamics simulations are performed to determine the molecular origins of charge-induced expansion in porous carbons.
Atomic force microscopy is used to monitor the expansion of porous carbon electrodes, which results from insertion/adsorption of ions in carbon pores during charging. The strain data collected at various potential scan rates are used to obtain information on anion and cation kinetics. Molecular dynamics simulations are performed to determine the molecular origins of charge-induced expansion in porous carbons.
The expansion of porous carbon electrodes in a room temperature ionic liquid (RTIL) is studied using in situ atomic force microscopy (AFM). The effect of carbon surface area and pore size/pore size distribution on the observed strain profile and ion kinetics is examined. Additionally, the influence of the potential scan rate on the strain response is investigated. By analyzing the strain data at various potential scan rates, information on ion kinetics in the different carbon materials is obtained. Molecular dynamics (MD) simulations are performed to compare with and provide molecular insights into the experimental results; this is the first MD work investigating the pressure exerted on porous electrodes under applied potential in a RTIL electrolyte. Using MD, the pressure exerted on the pore wall is calculated as a function of potential/charge for both a micropore (1.2 nm) and a mesopore (7.0 nm). The shape of the calculated pressure profile matches closely with the strain profiles observed experimentally.
Supporting Information
N2 adsorption measurements were performed to determine the surface area and pore size distribution s of the carbon membranes used in this study. Figure S1 a and b show the N2 adsorption isotherms as well as the calculated pore size distributions (PSDs) for the MC, MC - A, and MC - G membranes. These are type IV isotherms with H1 hysteresis loops characteristic of materials with large mesopores. [1]
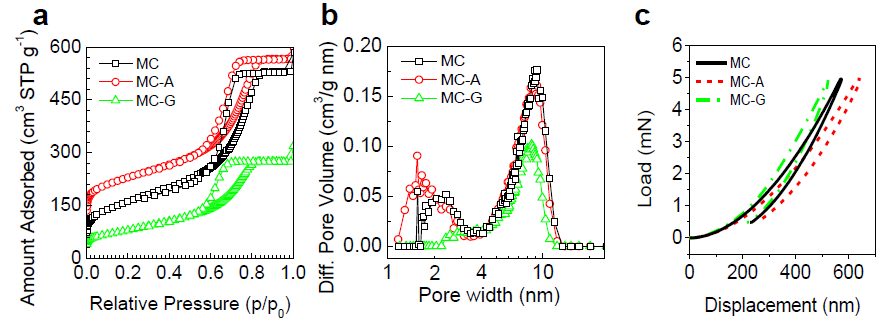
The steepness of the capillary condensation step results from the uniform diameter of the main mesopores and consequently narrow pore size distribution, which i s usually reported for ordered and disordered soft - templated carbon materials. The surface area of the MC, MC - A, and MC - G carbons w ere determined to be 579, 798, and 282 m 2 g - 1 , respectively. Mechanical indentation experiments were also performed to dete rmine the hardness of the carbon membranes, and results are shown in Figure S1c. From the indentation experiments the Young’s modulus for the MC, MC - A, and MC - G w ere determined to be 6.783, 4.586, and 9.655 GPa, respectively.
To compare the strain behavior of a non-porous carbon with the porous carbons used in this study, the charge induced expansion of a non-porous glassy carbon electrode was also measured using in-situ AFM.
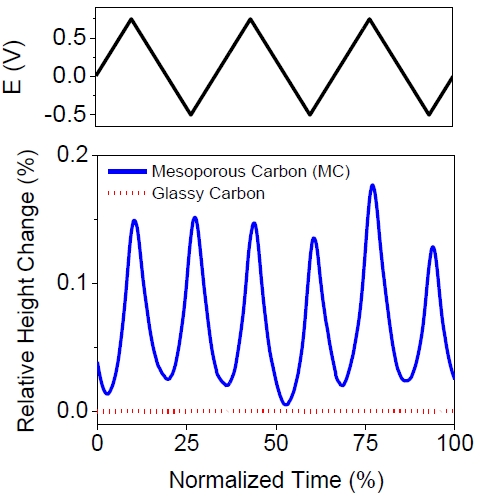
Figure S2 shows the relative height change of MC membrane and glassy carbon over three cyclic voltammogram cycles performed at 1 mV s-1. The MC membrane experiences an expansion of ca. 0.15% with the maximum occurring at the most positive and most negative applied potentials. For the glassy carbon electrode the maximum strain observed was very small (<0.001 %), and was unrelated to the potential applied to the electrode. The absence of strain observed in the non-porous glassy carbon provides further support that the expansion experienced in porous carbon materials is related to the ion insertion/adsorption in the carbon pores.
References: [1] X. Q. Wang, Q. Zhu, S. M. Mahurin, C. D. Liang, S. Dai, Carbon, 2010, 48, 557



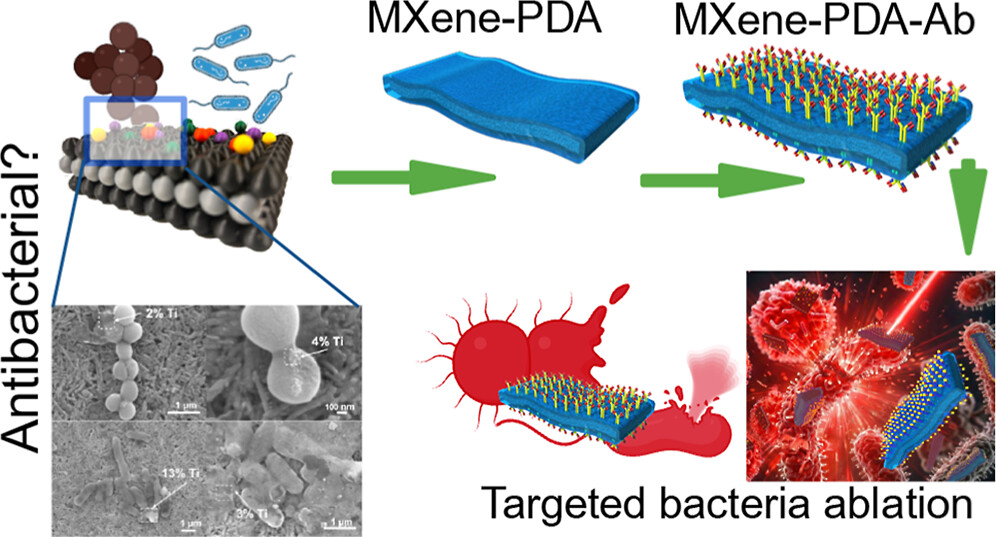 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.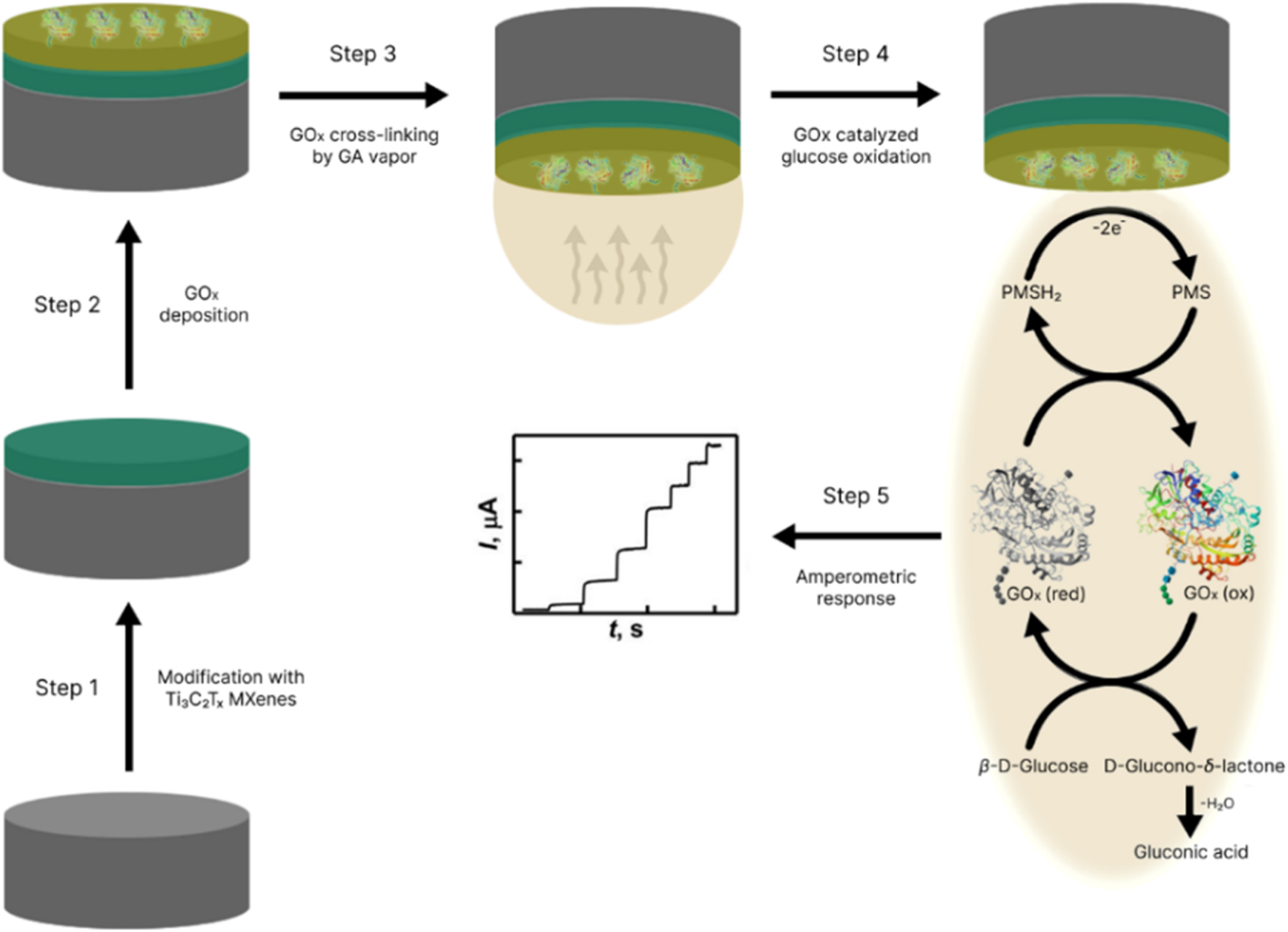
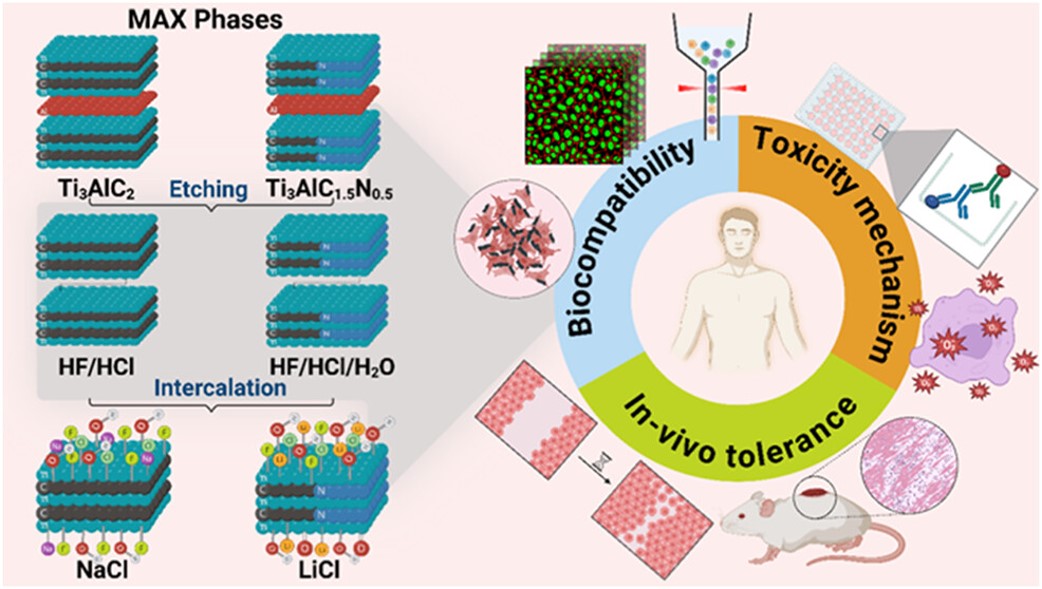 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.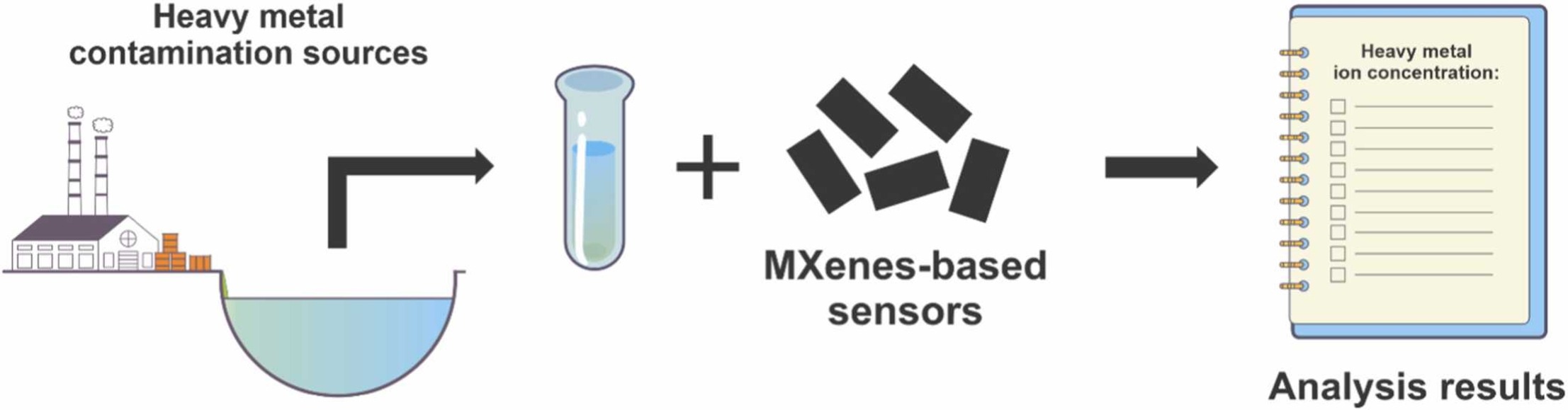 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!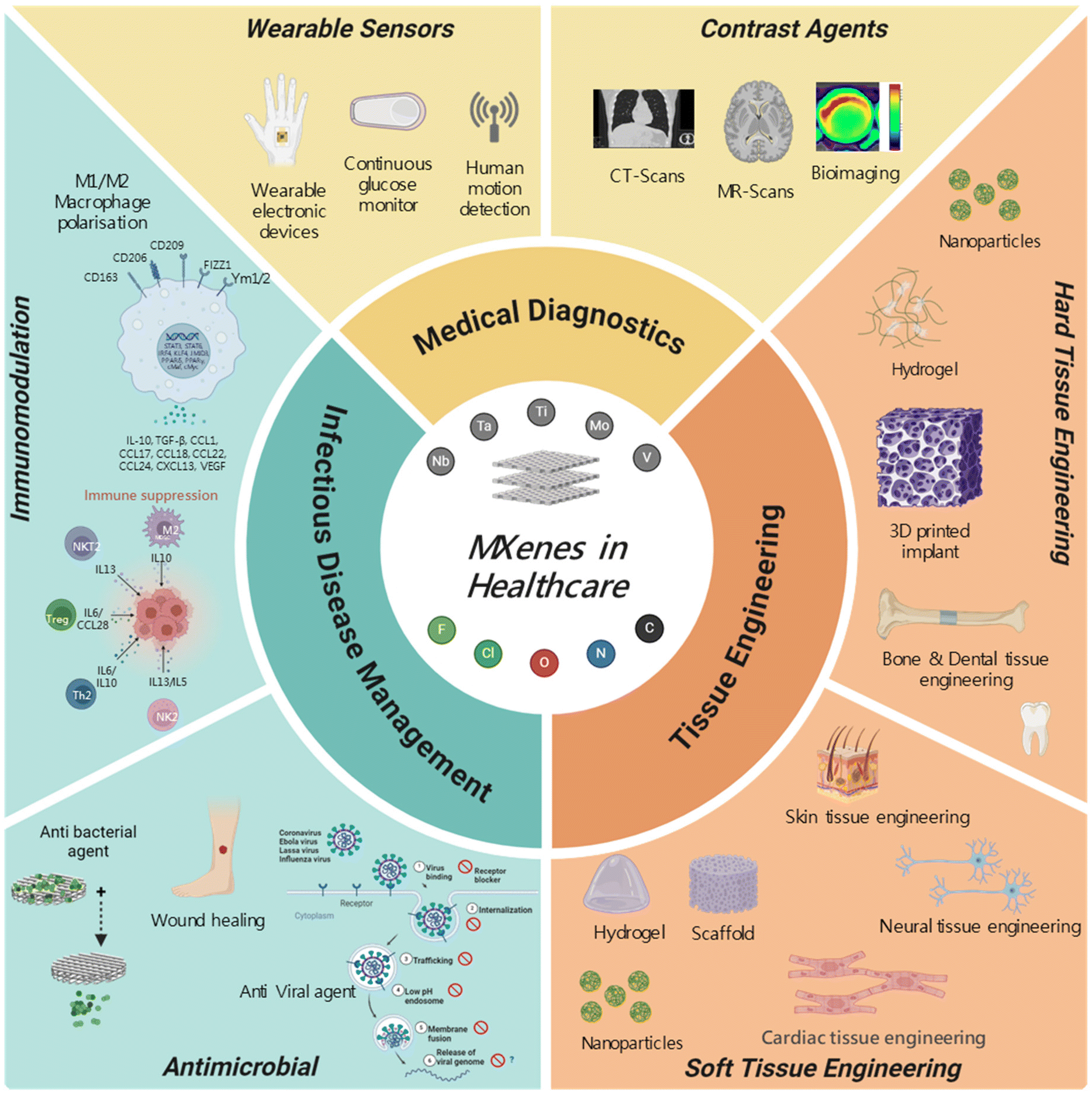 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.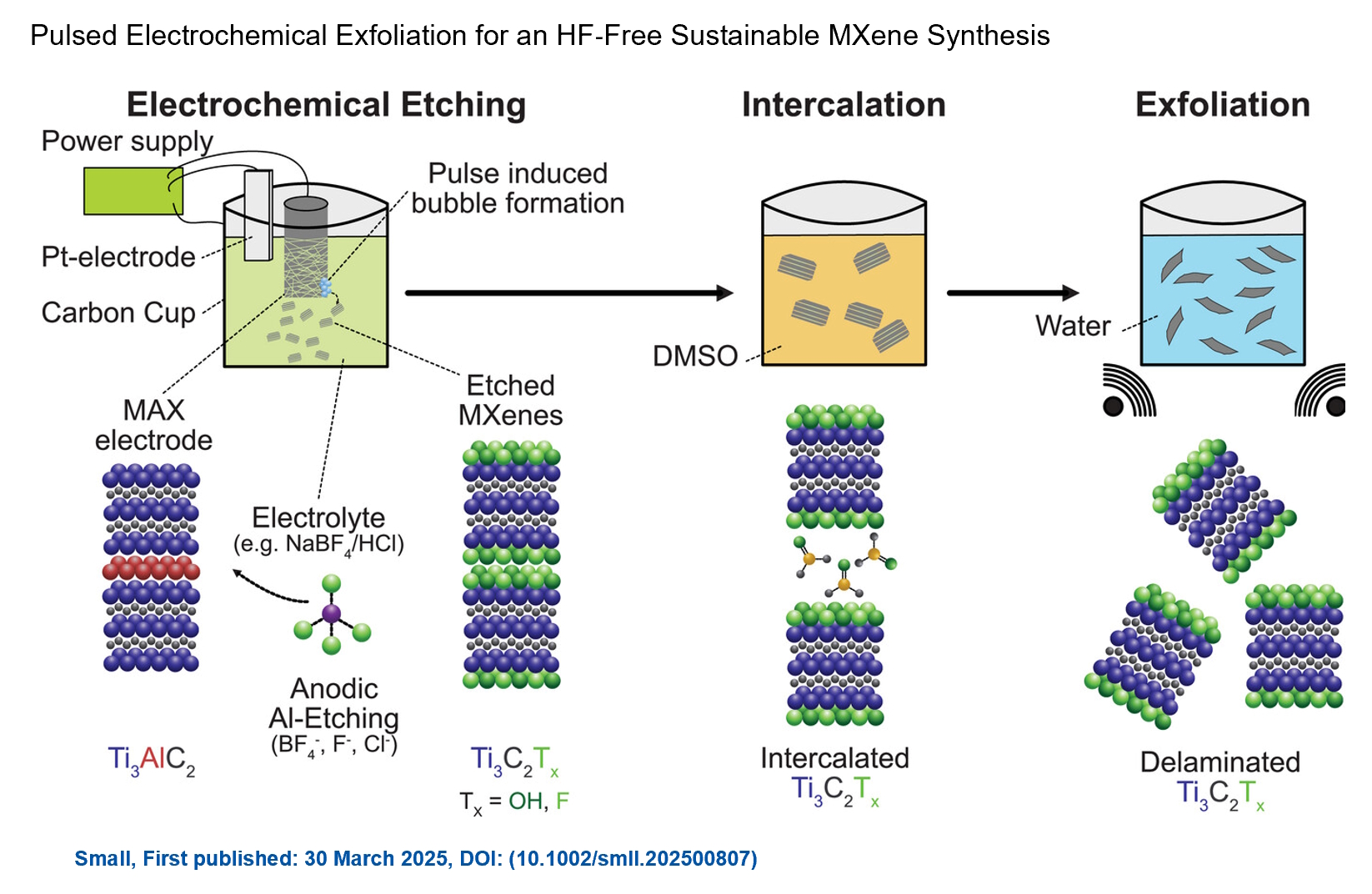 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.