| Rus |
 |
Eng |
 |
|

The 224th Electrochemical Society ECS Meeting was held in the heart of San Francisco, at the meeting headquarters hotel, the Hilton San Francisco (333 O’Farrell Street, San Francisco, CA 94102).
ECS bridges the gaps among academia, research, and engineering—bringing together scientists from around the world for the exchange of technical information. This unique blend provides an unparalleled forum for the integration of these areas of science and technology.
This major international conference at the Hilton San Francisco included more than 50 topical symposia consisting of over 2,800 technical presentations, and feature the third international ECS Electrochemical Energy Summit (E2S), which is fast becoming a tradition at ECS meetings. 2S and ECS Short Courses help launch the meeting on Sunday, October 27.
Two days of special events were devoted to E2S, with a featured symposium that explored the energy– water nexus, the intersection of two critical resource issues. Events on Sunday included an afternoon program with three invited speakers, all experts in energy issues, along with a dynamic Energy Research Group Showcase, a Poster Session, and a reception. E2S events on Monday were devoted to the Energy–Water Nexus Symposium (A3), and The ECS Lecture. Also at the conference was held Technical Exhibit and General Society and Poster Sessions.
On Monday, October 28, graduate research assistant from Nanomaterials group of Drexel University, Kelsey B. Hatzell, have presented at Poster Session presentation on Methods for Enhancing the Flowable Electrode Capacitance in the Electrochemical Flow Capacitor, resulting by common work of Drexel research team - Majid Beidaghi, Muhammed Boota, Christopher R Dennison, Emin Caglan Kumbur and professor Yury Gogotsi.
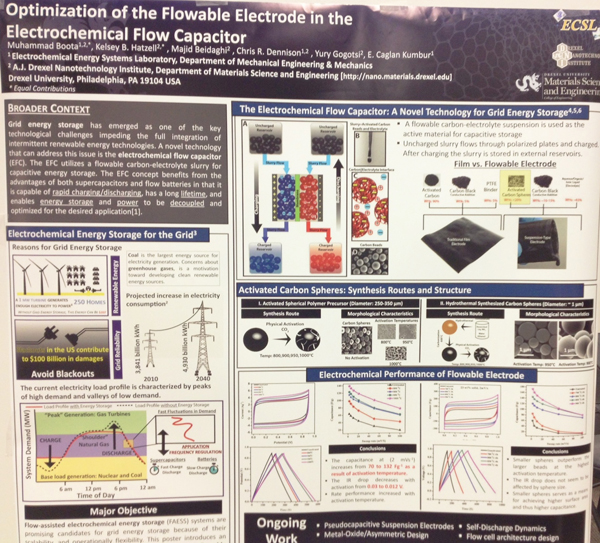
Methods for Enhancing the Flowable Electrode Capacitance in the Electrochemical Flow Capacitor
K.B. Hatzell1, M. Beidaghi1, M. Boota1,2, C.R.Dennison1,2, E.C. Kumbur2, Y. Gogotsi2
1 A.J. Drexel Nanotechnology Institute, Department of Material Science and Engineering
2 Electrochemical Energy Systems Laboratory, Department of Mechanical Engineering and Mechanics Drexel University, Philadelphia, PA 19104
Abstract
Grid energy storage has emerged as one of the key challenges limiting grid resiliency and impeding the full integration of intermittent renewable energy technologies. A novel technology that can address this issue is the electrochemical flow capacitor (EFC). The primary difference between traditional supercapacitors and the EFC is that the EFC utilizes a flowable electrode for capacitive energy storage. The electrostatic nature of this charge storage mechanism allows for fast charging and discharging, which allows the EFC to be tailored to specific grid applications such as voltage and frequency regulation where short response times are needed.
Researchers have investigated the effects of pore size distribution and ammonization of the carbon surface as possible methods to increase the capacitance of the active material in the flowable electrode.


On Wednesday, October 30, 2013 Kelsey B. Hatzell, graduate research assistant from Nanomaterials group, Drexel University, made a report on High Electrosorption Capacity Electrodes for Capacitive Deionization at the Energy–Water Nexus Symposium (A3) of ECS Electrochemical Energy Summit, at 224th ECS Meeting.
High Electrosorption Capacity Electrodes for Capacitive Deionization
Kelsey B. Hatzell1, Etsuro Iwama2, Barbara Daffos2, Pierre-Louis Taberna2, Theo Tzedakis2, Alexei Gogotsi3 , Patrice Simon2, Yury Gogotsi1
1 A.J. Drexel Nanotechnology Institute, Materials Science and Engineering Department, Drexel University, Philadelphia, PA
2 Université Paul Sabatier, CIRIMAT UMR CNRS 5085, 118 route de Narbonne, 31062 Toulouse, France
3 Materials Research Centre, 03680 Kiev, Ukraine
Abstract
In water stressed regions across the globe, the rate of abstraction from deep aquifers often exceeds the rate of recharge. This leads to water shortages that are sometimes irreversible. In order to address these water shortages, researchers are looking to the most abundant of source water present on earth, seawater. However, to transform seawater into clean drinking water requires a range of energy intensive processes. Such processes include Reverse Osmosis, UV disinfection and Thermal Distillation. The most promising of these technologies is Reverse Osmosis, which can achieve 1.8 kWh/m3 in current commercial plants [1]. Nevertheless, this technology is fundamentally hindered by membrane fouling and slow water transport [2]. Thus, there has been a movement toward technologies that do not use membranes, and toward technologies that remove the minority component (salt) rather than the majority component (water) [3].
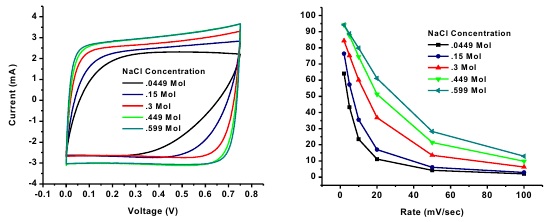
Capacitive Deionization (CDI) is the process of removing ions from brackish/seawater by applying a potential between two electrodes, adsorbing ion on the surface, and producing clean water. Carbon materials are favorable as electrode materials in CDI systems because they exhibit high electric conductivity (~100 S m-1), specific surface area (up to 2000 m2 g-1), and high electrochemical stability. Herein, we report the use of spherical activated carbon beads (BET SSA 1219 m2 g-1) as the active material for electrodes for a capacitive deionization system. In a 0.15 M solution of NaCl at 10 mV s-1 the electrodes demonstrate a capacitance of 58 F/g which is on par with recently reported electrode capacitances. These results indicate that with further optimization, the spherical geometry of the particles may yield enhanced electrosorption capacity for CDI.
References:
1. Elimelech, M.; Phillip, W.A. The Future of seawater desalination: Energy, technology, and the environment. Science 2011, 333-712-717.
2. Wang, Evelyn and Karnik, Rohit. Graphene Cleans up water. Nature Nanotechnology, 2012.
3. Porada, S., Weinstein, L., Dash, R., Van Der Wal, A., Bryjak, M., Gogotsi, Y., & Biesheuvel, P. M. Water desalination using capacitive deionization with microporous carbon electrodes. ACS Applied Materials & Interfaces, 4(3), 1194-1199, 2012.
Source: www.ecs.confex.com









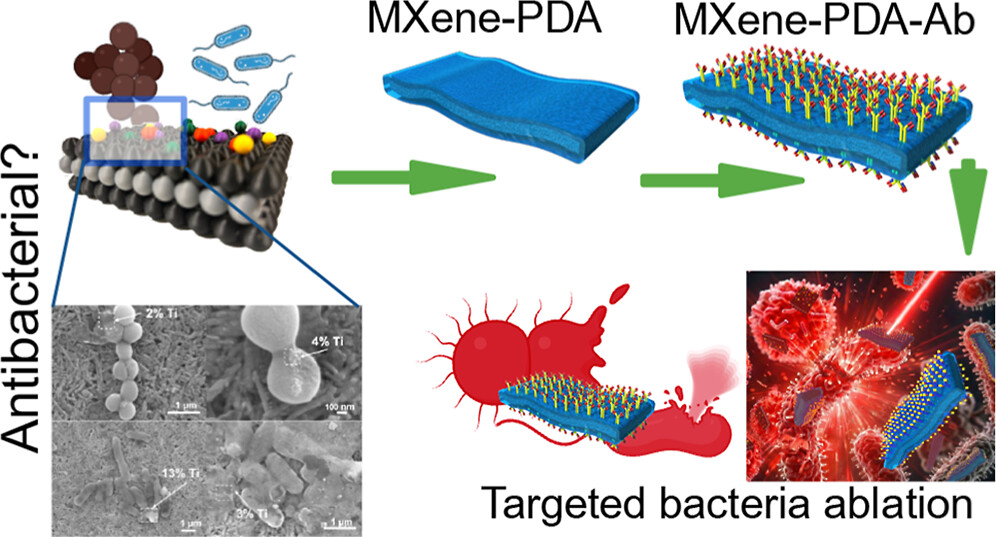 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.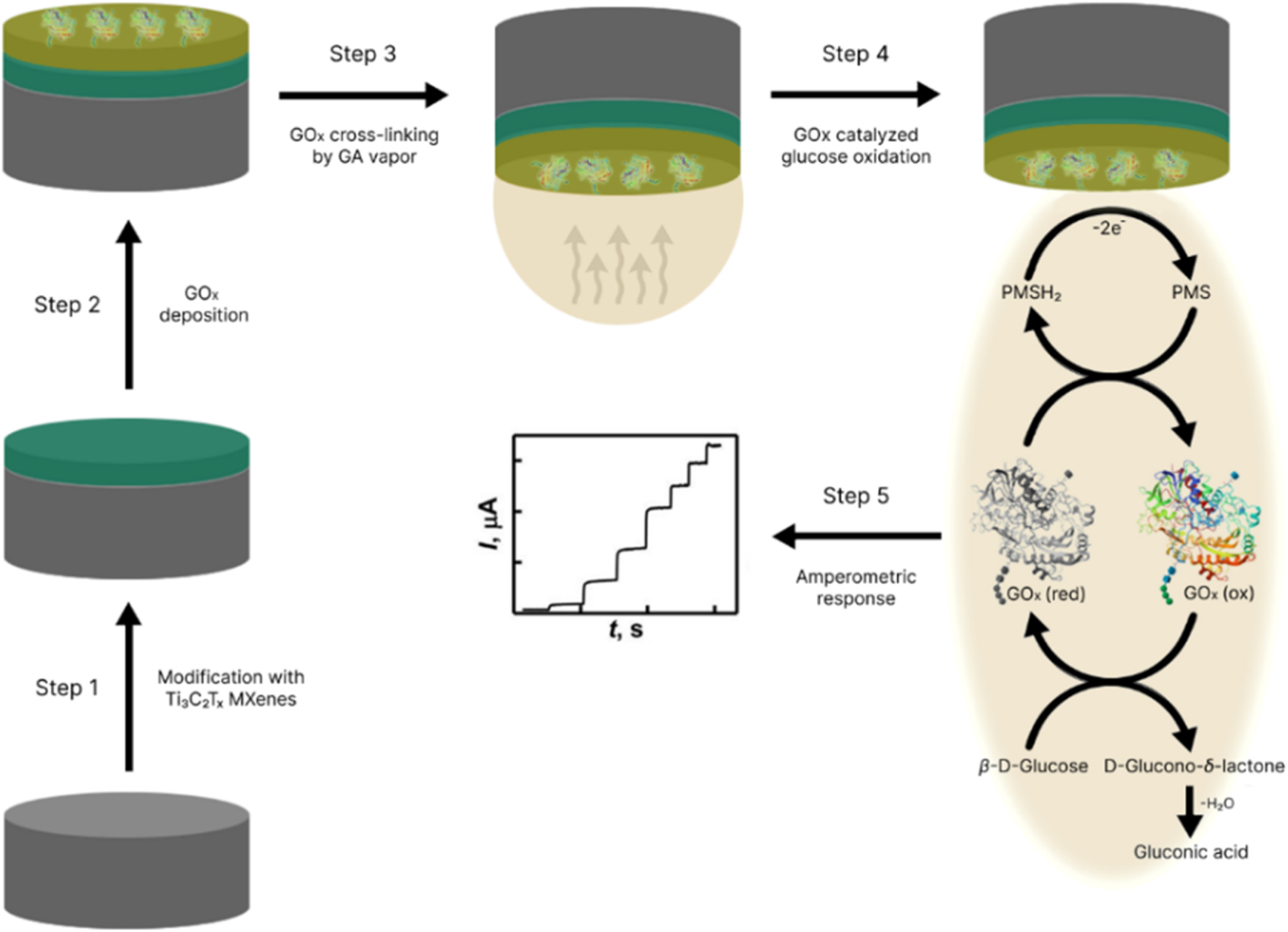
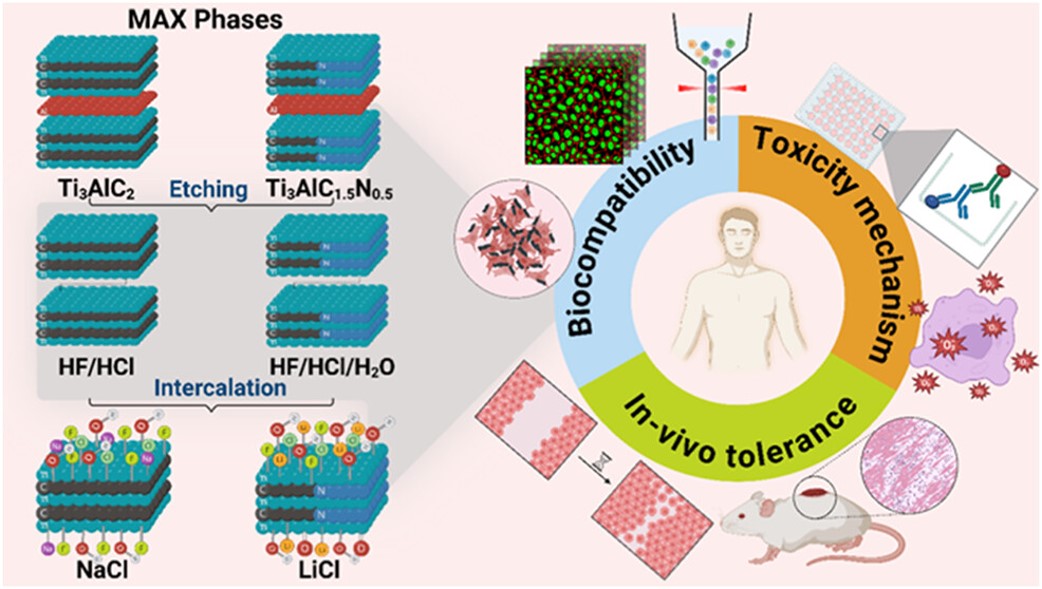 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.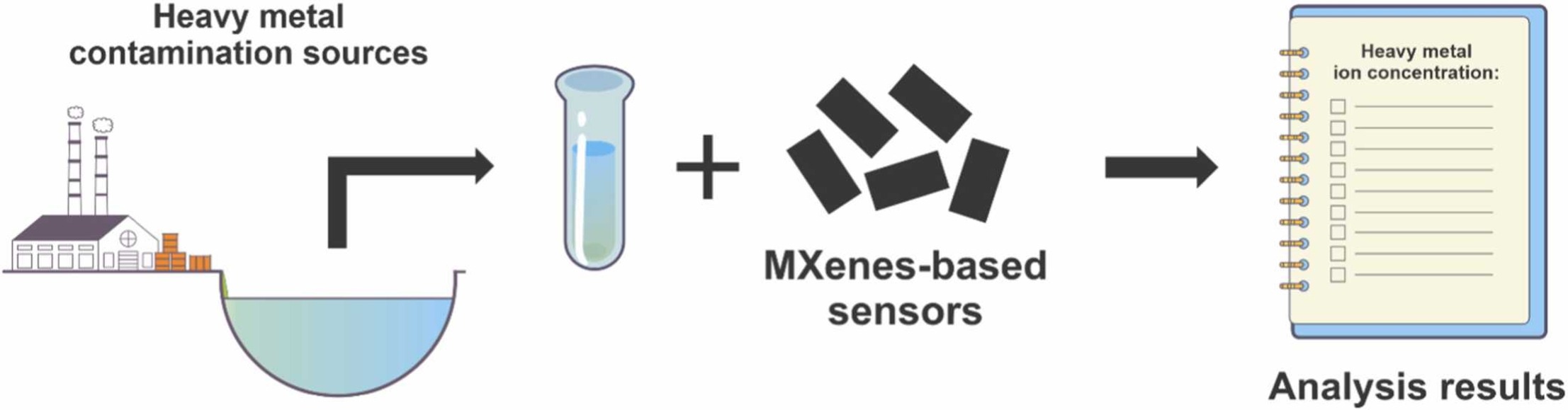 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!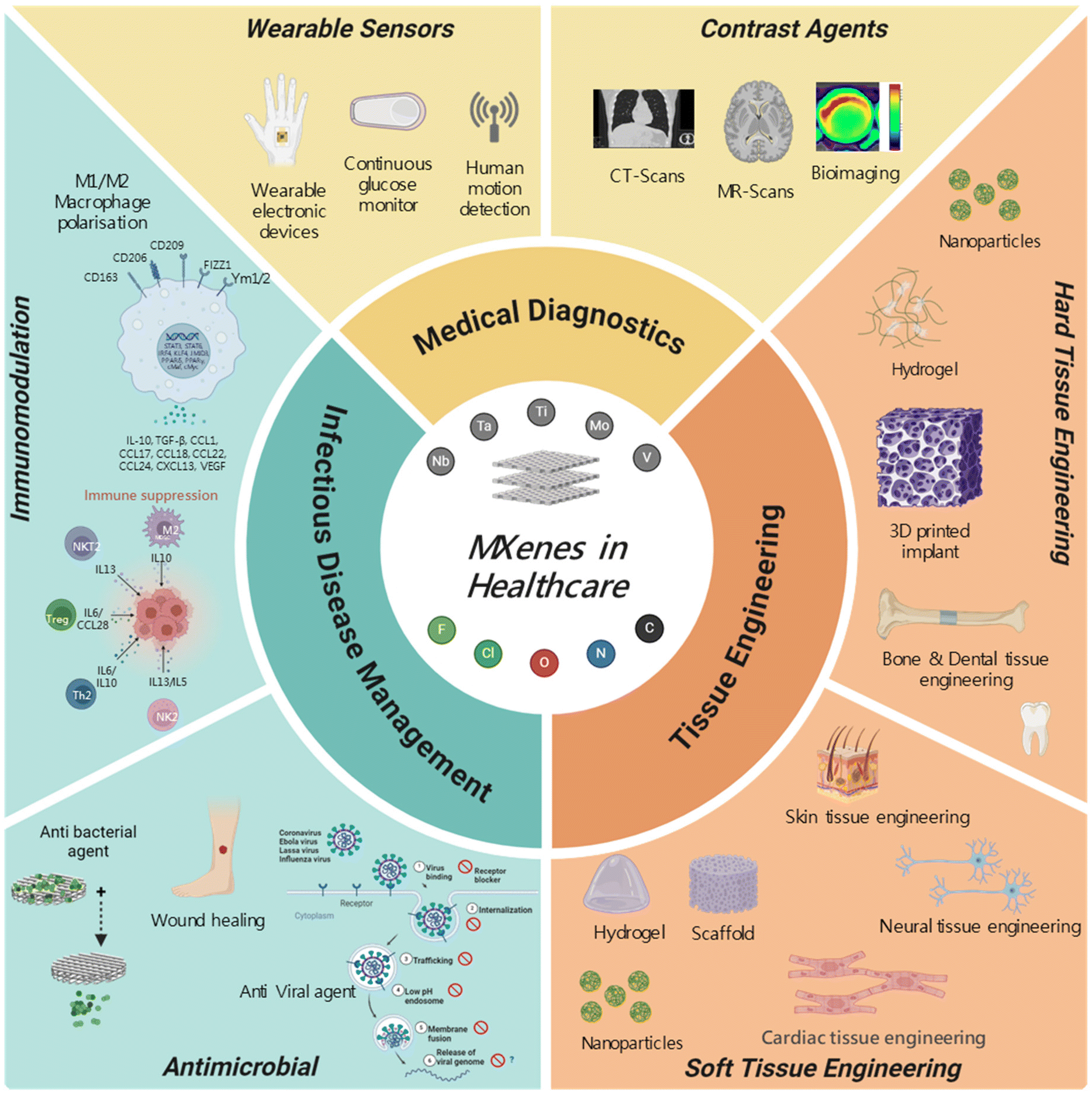 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.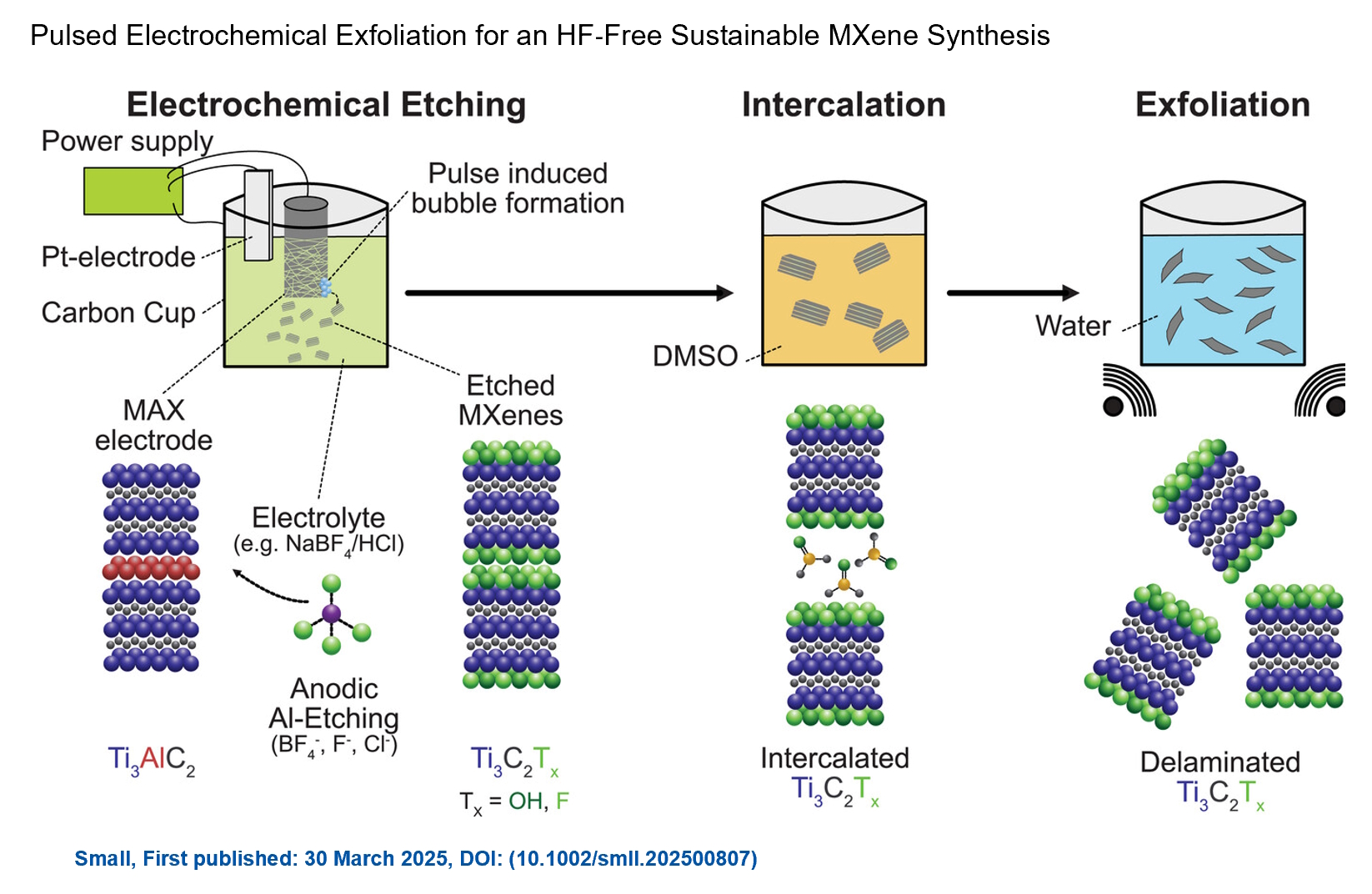 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.