| Rus | Eng |
Science 27 September 2013: Vol. 341 no. 6153 pp. 1502-1505. DOI: 10.1126/science.1241488
Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide
Maria R. Lukatskaya1,2, Olha Mashtalir1,2, Chang E. Ren1,2,*, Yohan Dall’Agnese1,2,3,4, Patrick Rozier3, Pierre Louis Taberna3, Michael Naguib1,2, Patrice Simon3,4, Michel W. Barsoum1, Yury Gogotsi1,2
1Department of Materials Science and Engineering, Drexel University, Philadelphia, PA 19104, USA.
2A. J. Drexel Nanotechnology Institute, Drexel University, Philadelphia, PA 19104, USA.
3Université Paul Sabatier, CIRIMAT UMR CNRS 5085, 118 route de Narbonne, 31062 Toulouse, France.
4Réseau sur le Stockage Electrochimique de l’Energie (RS2E), FR CNRS 3459, France.
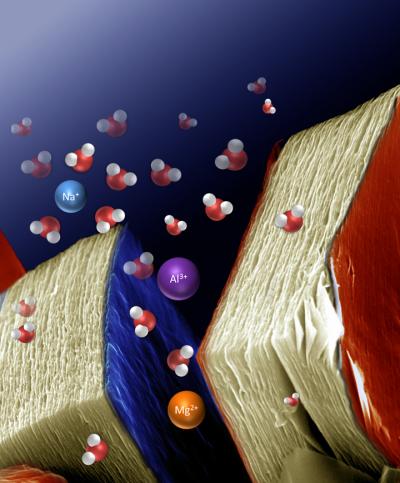
Many batteries and capacitors make use of lithium intercalation as a means of storing and transporting charge. Lithium is commonly used because it offers the best energy density, but also because there are difficulties in storing larger cations without disrupting the crystal structure of the host. Lukatskaya et al. developed a series of MX compounds, where M represents a transition metal and X is carbon or nitrogen.The compound Ti3C2 forms a two dimensional layered structure, which is capable of accommodating a wide range of cations, including multivalent ones, either spontaneously or electrochemically.
The intercalation of ions into layered compounds has long been exploited in energy storage devices such as batteries and electrochemical capacitors. However, few host materials are known for ions much larger than lithium. Researchers demonstrated the spontaneous intercalation of cations from aqueous salt solutions between two-dimensional (2D) Ti3C2 MXene layers. MXenes combine 2D conductive carbide layers with a hydrophilic, primarily hydroxyl-terminated surface. A variety of cations, including Na+, K+, NH4+, Mg2+, and Al3+, can also be intercalated electrochemically, offering capacitance in excess of 300 farads per cubic centimeter (much higher than that of porous carbons). This study provides a basis for exploring a large family of 2D carbides and carbonitrides in electrochemical energy storage applications using single- and multivalent ions.
About three years ago, Dr. Michel W. Barsoum and Dr. Yury Gogotsi, professors in Drexel’s College of Engineering, discovered atomically thin, two-dimensional materials -similar to graphene- that have good electrical conductivity and a surface that is hydrophilic, or can hold liquids. They named these new materials “MXenes,” which hearkens to their genesis through the process of etching and exfoliating atomically thin layers of aluminum from layered carbide “MAX phases.” The latter also discovered at Drexel about 15 years ago by Barsoum
Since then, the pair, and their team of materials scientists, have forged ahead in exploring the potential uses of MXenes. Their latest findings are reported in the Sept. 27 issue of Science. In their piece entitled “Cation Intercalation and High Volumetric Capacitance of Two-dimensional Titanium Carbide,”Gogotsi and Barsoum along with Drexel researchers Maria Lukatskaya, Olha Mashtalir, Chang Ren, Yohan Dall’Angese and Michael Naugib and Patrick Rozier, Pierre Louis Taberna and Dr. Patrice Simon from Université Paul Sabatier in France, explain how MXenes can accommodate various ions and molecules between their layers by a process known as intercalation.
Intercalation is sometimes a necessary step in order to exploit the unique properties of two-dimensional materials. For example, placing lithium ions between the MXene sheets makes them good candidates for use as anodes in lithium-ion batteries. The fact that MXenes can accommodate ions and molecules in this way is significant because it expands their ability to store energy.
“Currently, nine MXenes have been reported by our team, but there are likely many more that will be discovered - the MXene-and-ion combinations that have been tested to date are by no means an exhaustive demonstration of the material’s energy storage capabilities,” said Gogotsi, who is also director of the A.J. Drexel Nanotechnology Institute. “So even the impressive capacitances that we are seeing here are probably not the highest possible values to be achieved using MXenes. Intercalation of magnesium and aluminum ions that we observed may also pave the way to development of new kinds of metal ion batteries.”
Barsoum and Gogotsi’s report looks at intercalation of MXenes with a variety of ions, including lithium, sodium, magnesium, potassium, ammonium and aluminum ions. The resulting materials show high energy storage capacities and present another avenue of research in this branch of materials science.
“Two-dimensional, titanium carbide MXene electrodes show excellent volumetric super capacitance of up to 350 F/cm3 due to intercalation of cations between its layers,” Barsoum said. “This capacity is significantly higher than what is currently possible with porous carbon electrodes. In other words, we can now store more energy in smaller volumes, an important consideration as mobile devices get smaller and require more energy”
The researchers also reported on using MXene “paper” electrodes, instead of conventional rolled powder electrodes with a polymer binder. The flexibility of this paper suggests MXenes may also be useful in flexible and wearable energy storage devices, which is another major area of ongoing research at Drexel in collaboration with Professor Genevieve Dion’s Shima Seiki Haute Technology Laboratory.
Source: www.esciencenews.com
RELATED ITEMS:
The research reported in this paper is an exciting advance in this new family of materials for which the applications are just beginning to be envisioned




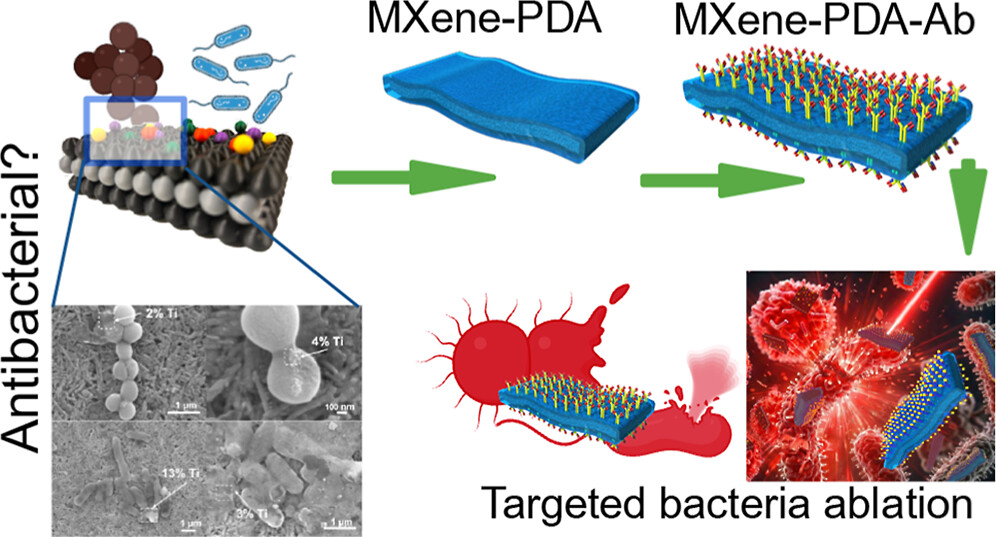 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
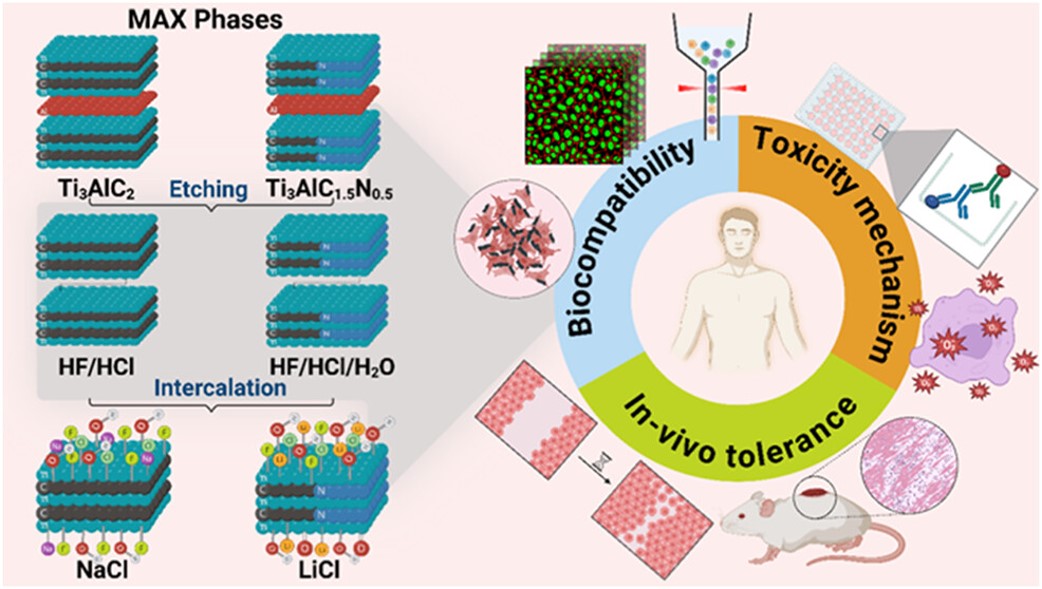 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.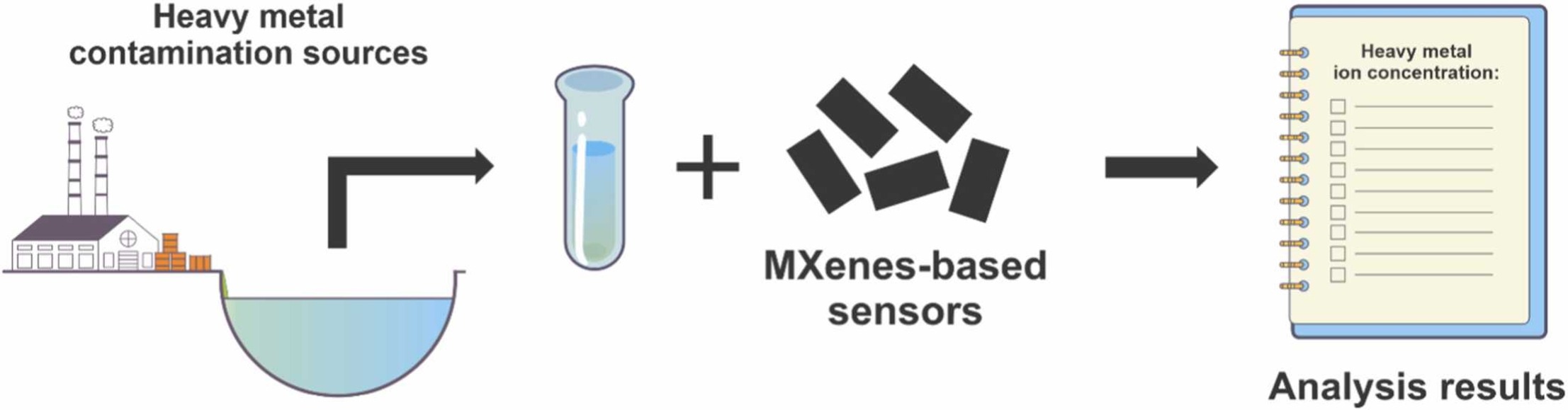 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!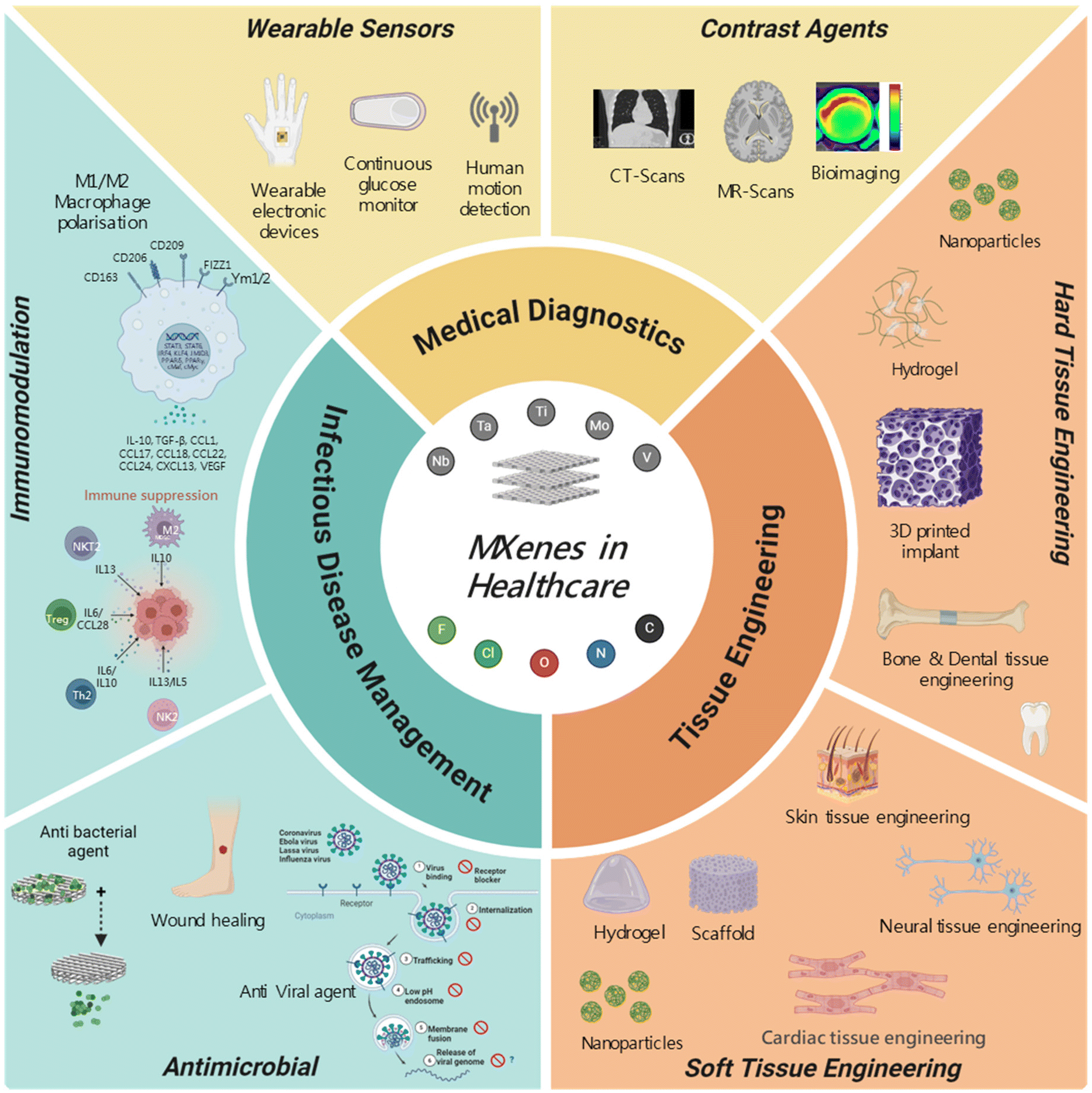 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.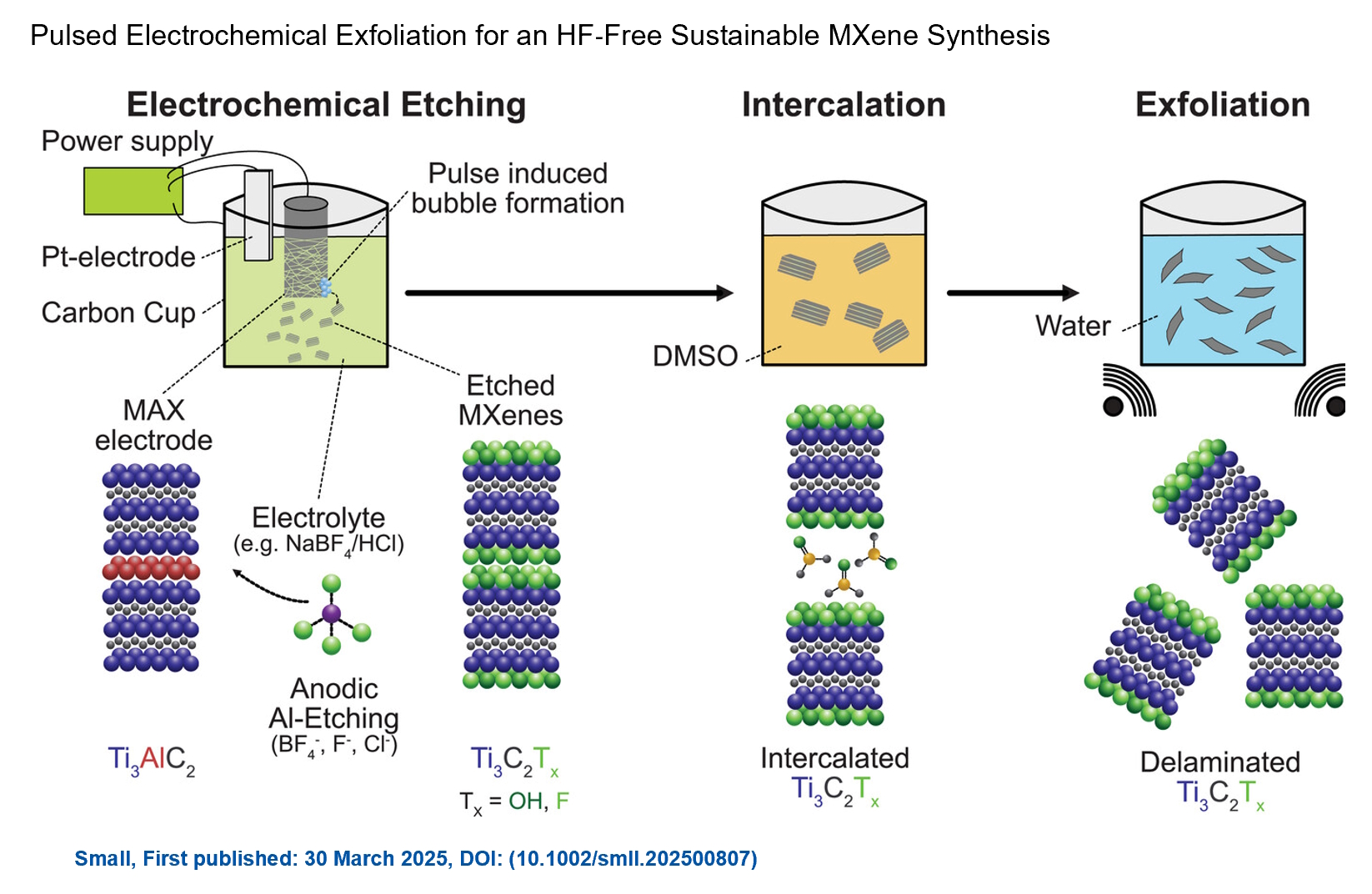 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.