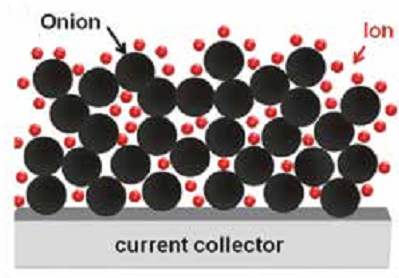
 Carbon onions represent one of the least studied carbon nanomaterials, and are seeing a large increase in attention for energy storage applications.
Carbon onions represent one of the least studied carbon nanomaterials, and are seeing a large increase in attention for energy storage applications.
| Rus | Eng |
J.K. McDonough, Y. Gogotsi, Carbon Onions: Synthesis and Electrochemical Applications, Interface, Fall 2013, 61-66 (2013)
Beginning with fullerenes, moving to carbon nanotubes, and most recently to graphene, carbon nanomaterials are widely studied and used in a range of applications including electronics, tribology, and energy storage. However, two kinds of carbon nanoparticles, nanodiamond1 and carbon onions, 2 which were discovered before fullerenes and nanotubes, stayed for a long time in the shadow of more popular and better investigated nanocarbons. However, both have become increasingly studied in recent years. Carbon onions consist of spherical closed carbon shells and owe their name to the concentric layered structure resembling that of an onion. Carbon onions are sometimes called carbon nano-onions (CNOs) or onion-like carbon (OLC). Those names cover all kinds of concentric shells, from nested fullerenes to small (<100 nm) polyhedral nanostructures. This review is dedicated to those materials. We first discuss the structure of carbon onions and provide an overview of their synthesis methods. Also, electrochemical applications of carbon onions are reviewed with an emphasis on supercapacitor electrodes.
Sumio Iijima discovered OLC in 1980 while looking at a sample of carbon black in a transmission electron microscope. OLC was not produced in bulk, but rather was observed as a byproduct of carbon black synthesis. 3 About a decade later in 1992, Daniel Ugarte put forth a formation mechanism for the spherical graphitic structure. By focusing an electron beam on a sample of amorphous carbon, he was able to observe the formation of OLC in situ. Under an electron beam, the amorphous carbon graphitizes and begins to curl, and after sufficient time, the graphitic carbon closes on itself, forming an onion. The curving and closure occurs in order to minimize the surface energy of the newly formed edge planes of graphite, which is about 30x that of the basal plane. 4
Synthesis of Carbon Onions
Although OLC has been synthesized by many different methods in the last 30 years, large scale production (gram quantities) of OLC was first realized in 1994 by Vladimir Kuznetsov and co- workers, who used vacuum annealing of a nanodiamond precursor. 5,6 Similar to vacuum annealing, other groups have also utilized annealing in inert gases to transform nanodiamond, which is currently produced in ton quantities, 1 to OLC. 7 This is one of the methods that has a potential for industrial applications, as the onion yield is close to 100% and the manufacturing volume is only limited by the size of the furnace, and can be scaled accordingly. This material rarely has ideal spherical carbon onions, but can be produced in large quantities and finds practical applications. The transition of nanodiamond to a carbon onion can be seen in a molecular dynamics (MD) simulation (Fig. 1a-c). A 2-nm particle of nanodiamond (Fig. 1a) was annealed at 1400 °C (Fig. 1b) causing the outer layers of the nanodiamond to convert to graphitic carbon; however the annealing was not at high enough temperature to convert the entire particle. At higher temperatures (Fig. 1c), the entire particle is converted to an OLC particle. 8 At the highest annealing temperatures, the OLC particle begins to polygonize (Fig. 1d) as the structure becomes more ordered. The particle size of OLC produced via nanodiamond annealing is dependent on the nanodiamond precursor, which is generally about 5 nm in diameter, 1 producing onions in the 5-10 nm size range.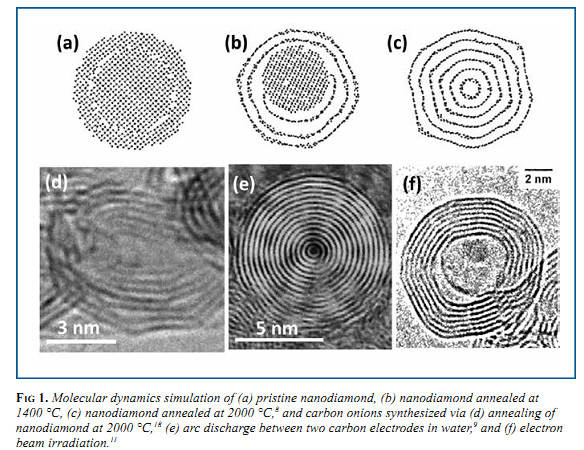
Arc discharge between two graphite electrodes in water represents another synthesis technique, generating OLC of slightly different structure than from annealing of nanodiamond. A dc current of 30 A and 17 V was applied between two graphite electrodes in water causing the carbon to evaporate at the location of the arc due to the extreme heat generated. The carbon vapor rapidly condenses into highly spherical OLC particles (Fig. 1e) and will float on the water surface, waiting to be collected for analysis. Consumption of the anode was about 100 mg/min, with the carbon products being produced at 20 mg/min. Synthesis by arc discharge can be performed at ambient pressure and temperature, avoiding the use of expensive equipment or catalysts, however the yield is low and samples contain nanotubes and amorphous carbon formed along with carbon onions. 9,10
Hollow carbon onions have been produced with the aid of metal nanoparticles. First, the metal and carbon were evaporated by an arc discharge method, similar to the one described earlier. The resulting product is a metal particle encapsulated by layers of graphitic carbon. When the system is exposed to the beam of a transmission electron microscope (TEM), the metal particle migrates a few atoms at a time through the carbon layers, which can be seen in situ, and leaves a hollow OLC particle (Fig 1f). 11 Laser excitation of ethylene causes the gas to decompose and produces solid carbon at high temperatures. The process, used by Gao, et al., is performed in air and uses a high-energy laser to convert the hydrocarbon to a solid carbon onion. This has potential to be used for large-scale production, as it can be scaled up, with authors showing a synthesis rate of 2.1 g/hour. This, along with annealing of detonation nanodiamond, is another feasible synthesis method for industry. 12
There are several other processes that were reported to produce carbon onions. Synthesis of carbon onions via chemical vapor deposition (CVD) utilized an iron catalyst supported on sodium chloride to decompose acetylene gas at temperatures ~400 °C. For less than 5 wt% iron, the carbon onions had an Fe3 C core. In contrast to other methods of carbon onion synthesis, this CVD process yields much larger diameter particles, ~50 nm. 13 Carbon ion implantation is another method to produce carbon onions, first used in 1998 by Cabioc’h, et al., which allowed the particle diameter to be tuned from 3 nm up to 30 nm by varying synthesis conditions such as temperature or implantation dose density. 14 Thermolysis is when a compound is decomposed by heat and has been shown to be a method for carbon onion synthesis. Using sodium azide (NaN3 ) and hexachlorobenzene (C6 Cl 6 ) as the reagents, a redox reaction causes an abrupt increase in temperature and pressure, producing large diameter carbon onions (30 - 100 nm) and other impurities, such as sodium chloride and amorphous carbon, which can be removed through a purification step. 15 Solid state carbonization of a phenolic resin precursor is a way to produce larger diameter carbon onions, ~40 nm. The precursor material was a phenol-formaldehyde resin and required the aid of a ferric nitrate catalyst at temperatures ~1000 °C. 16 High temperature evaporation of nanodiamond resulted in carbon condensing on a silicon substrate, with the carbon having the form of onions. The resulting particles had a diameter ~5 nm. This paper does not show any information regarding mass or yield, and it seems like a low yield process. 17
Structure of Carbon Onions
The onions consist of graphene shells with pentagonal and other defects required to have closed-shell structures. Structural properties of OLC vary significantly depending on the synthesis conditions. Focusing on OLC derived from the annealing of nanodiamond between 1300 and 1800 °C, the BET specific surface area (SSA) from N2 gas adsorption varies between 400 and 600 m2 /g (Fig. 2a). There is no accessible internal porosity for OLC, so the SSA is dependent on the density of the material and the surface of the particles. At lower annealing temperatures, there is residual diamond in the sample causing a lower SSA due to a higher density of nanodiamond compared to graphitic carbon forming onion shells. A maximum at 1500 °C is found after all diamond is transformed to OLC and the particle has a rough (defective) surface, and the subsequent decrease in SSA is due to sintering and formation of larger polygonized particles as annealing temperature in increased. The pore size distribution of OLC is broad in the mesoporous range, as any “pore” is actually formed by the space between multiple onions, and does not vary significantly between synthesis conditions. 18
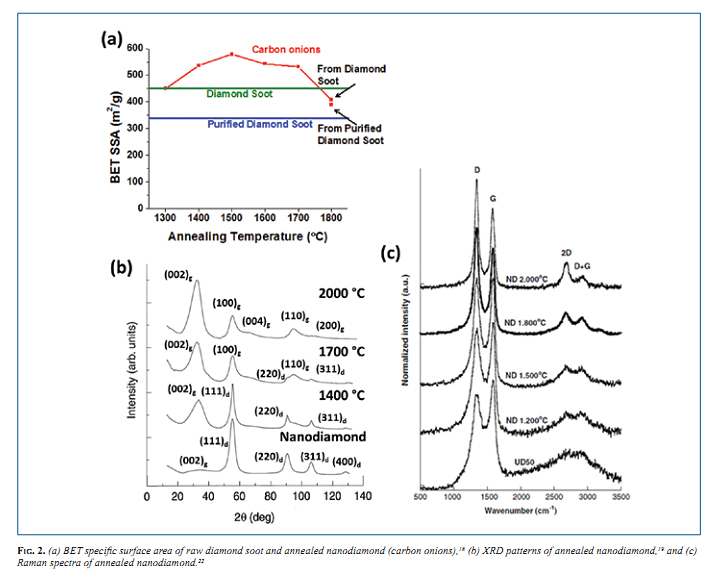
Conversion from nanodiamond to fully graphitized onions was investigated using X-ray diffraction (XRD) (Fig. 2b). The nanodiamond precursor shows pronounced diamond peaks, as expected, in addition to a small peak for (002) graphite. Upon annealing for 30 minutes at 1400 °C, three peaks appear for graphite that are relatively broad and weak in intensity, probably because the graphitic carbon is still defective and incomplete shells are formed. The graphitic carbon peaks grow for the sample annealed at 1700 °C, with some residual diamond peaks. Finally, after annealing at 2000 °C for 30 minutes, no diamond peaks are present, with very pronounced peaks for graphite. 19,20
Raman spectroscopy was used by Portet, et al. to study the surface structure of carbon onions as they are annealed from nanodiamond at temperatures between 1200 and 2000 °C (Fig. 2c). The nanodiamond precursor (UD50 grade1 ) used was a detonation nanodiamond soot, that is comprised of disordered carbon, carbon onions, and diamond nanoparticles, with ~25 wt% of sp3 carbon and ~75 wt% of sp2 carbon. 21 The Raman spectra for all samples contain two peaks at 1350 and 1600 cm-1 , corresponding to the D-band for disordered carbon and the G-band for graphitic carbon, respectively, in addition to second order peaks for the 2D band at ~2700 cm-1 and for the G+D band at ~2850 cm-1 . The spectrum for UD50 shows two very broad D and G peaks, and the 2D and D+G peaks are unresolvable, implying a highly disordered graphitic carbon present in the detonation soot. Annealing the nanodiamond at 1200 °C causes a narrowing of all peaks, and appearance of the resonant peaks. As the samples are annealed at higher temperatures, up to 2000 °C, the peaks continue to narrow as the sp3 carbon and disordered carbon is further graphitized. The ratio of the D to G band (ID/I G, not shown) decreases significantly upon annealing due to the increase in ordering of the carbon particles. 22
Electrochemical Applications of Carbon Onions
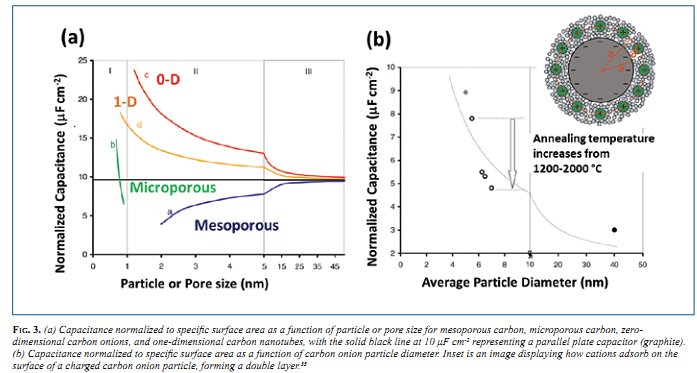
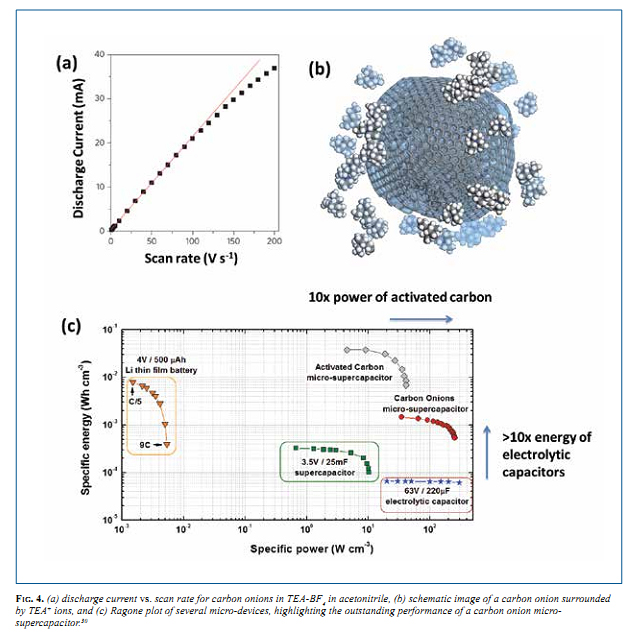
Electric double layer capacitors (EDLC), also known by their commercial names as supercapacitors or ultracapacitors, are non- faradaic electrical energy storage devices that store charge on the surface of a high surface area carbon electrode, often made of porous activated carbon. 23,24 Materials for supercapacitors range from microporous and mesoporous carbons, to one-dimensional carbon nanotubes, to two-dimensional graphene. The theoretical capacitance per surface area of these materials as a function of pore size and particle size is shown in Fig. 3a. Below a pore size or particle size of ~5 nm, the normalized capacitance deviates from planar graphite, with mesoporous materials decreasing in capacitance and materials with a positive curvature, i.e., carbon onions, increasing significantly. This figure shows that the smallest 0-D carbon onions can potentially outperform other materials in terms of capacitance per area. Carbon onions debuted as a material for EDLCs in 2006-2007 in both aqueous25,26 and organic22 electrolytes. Since then, there has been an immense amount of attention given to OLC for both batteries27-29 and supercapacitors, 18,22,30-34 as both active materials and easily dispersible conductive additives (ultimate carbon black). The high power capabilities of carbon onions, with excellent capacitance retention at current densities as high as 200 mA/cm2 (15 A/g) have been highlighted in the very first paper. 22 The theoretical values from Fig. 3a compared to published data in Fig. 3b show a very good agreement and an increase in capacitance as onion size decreases. 35 A few years later, in 2010, the carbon onion micro- supercapacitor was fabricated and tested in comparison to other systems at the same length scale. 30 The micro-supercapacitor using interdigital OLC electrodes was able to operate efficiently at rates as high as 100 V/s (Fig. 4), much faster than conventional EDLCs operating at rates well below 1 V/s. A plot of the discharge current vs. scan rate derived from cyclic voltammetry should be linear for a capacitive system and will deviate at high enough rates due to diffusion limitations of the ions in electrolyte. From Fig. 4a, the OLC micro-supercapacitor has a linear relationship up to about 100 V/s. Tetraethylammonium ions at the surface of a carbon onion particle are shown in Fig. 4b. The performance of other systems in comparison with the OLC micro- supercapacitor is shown in a Ragone plot (Fig. 4c). Carbon onions have roughly 10x the power of activated carbon, however a lower energy density because of the lower surface area. Electrolytic capacitors have a comparable or higher power density, yet carbon onions have more than 10x the energy density. 30
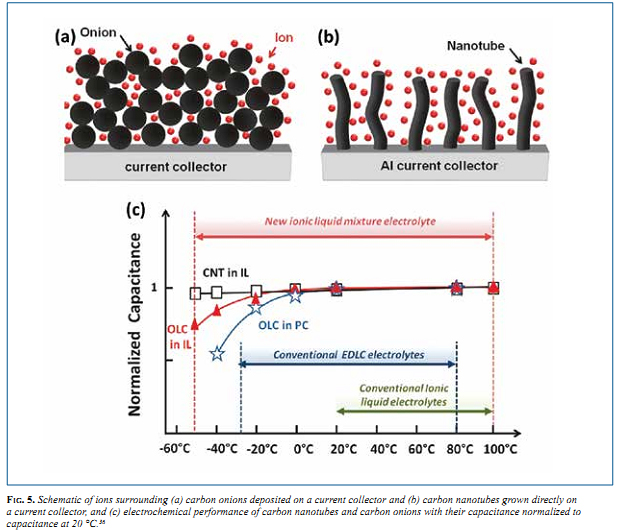
Recently, OLC and carbon nanotubes (CNTs) were used to store energy from -50 to 100 °C—a wider temperature range than any porous activated carbons can deliver with organic or aqueous electrolytes. The exohedral carbons were combined with a eutectic mixture of ionic liquids, which remains liquid at temperatures down to -80 °C. The arrangement of ions around the respective electrode materials can be seen in Fig. 5a and 5b. Both, carbon onions and nanotubes were found to operate efficiently at temperatures as low as -50 °C and as high as +100 °C. Conventional EDLC electrolytes utilize propylene carbonate (PC) as the solvent and begin to see a decrease in performance below 0 °C (Fig. 5c). Activated carbon was used in the same eutectic mixture of ionic liquids and failed at temperatures of -20 °C, even at slow charge- discharge rates. 36 This shows that adsorption of ions on the exohedral surfaces of onions (Fig. 5a) minimizes ion transport limitations allowing either very fast charge-discharge rates (Fig. 4a) or use of electrolytes with low mobility (Fig. 5c).
Conclusions
Carbon onions represent one of the least studied carbon nanomaterials, and are seeing a large increase in attention for energy storage applications. Because of their unique 0-D structure, small (<10 nm) diameter, high electrical conductivity, and relatively easy dispersion, compared to 1-D nanotubes and 2-D graphene, OLC has been shown to be ideal as a conductive additive to battery and supercapacitor electrodes, or as active material for supercapacitor electrodes for high-power applications and for low temperature devices using ionic liquid electrolytes.
Acknowledgments
This work was supported as part of the Fluid Interface Reactions, Structures and Transport (FIRST) Center, an Energy Frontiers Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences. The authors are thankful to Dr. Vadym Mochalin (Drexel University) for Fig. 4b.
About the Authors
John K. McDonough received his BS in Physics in 2009 from Lycoming College. Presently, he is a PhD candidate at Drexel University, Philadelphia, in the Department of Materials Science and Engineering, studying under the guidance of Yury Gogotsi. His research focuses on carbon nanomaterials for electrical energy storage, specifically on onion-like carbon for electrochemical capacitors. McDonough serves as the President of the MRS Chapter at Drexel and is also highly involved in the ECS and ASM Chapters.
Yury Gogotsi is a Distinguished University Professor and Trustee Chair of Materials Science and Engineering at Drexel University in Philadelphia. He also serves as Director of the A. J. Drexel Nanotechnology Institute. His PhD is in Physical Chemistry from Kiev Polytechnic and he holds a DSc in Materials Engineering from the Ukrainian Academy of Sciences. His research group works on nanostructured carbons, two-dimensional carbides, and their electrochemical applications. He has co- authored more than 300 journal papers. He is a Fellow of ECS, AAAS, MRS, ACerS, and a member of the World Academy of Ceramics. He may be reached at Этот e-mail адрес защищен от спам-ботов, для его просмотра у Вас должен быть включен Javascript .
References
1. V. N. Mochalin, O. Shenderova, D. Ho, and Y. Gogotsi, Nature Nanotechnol., 7, 11 (2012).
2. Yu.V. Butenko, L. Siller, and M. R. C. Hunt, in Carbon Nanomaterials, 2nd edition, Y. Gogotsi, V. Presser, Eds., CRC Press, pp. 279-231 (2013).
3. S. Iijima, J. Cryst Growth, 50, 675 (1980).
4. D. Ugarte, Nature, 359, 707 (1992).
5. V. L. Kuznetsov, A. L. Chuvilin, Y. V. Butenko, I. Y. Malkov, and V. M. Titov, Chem. Phys. Lett., 222, 343 (1994).
6. V. L. Kuznetsov, A. L. Chuvilin, E. M. Moroz, V. N. Kolomiichuk, S. K. Shaikhutdinov, Y. V. Butenko, and I. Y. Malkov, Carbon, 32, 873 (1994).
7. J. Cebik, J. K. McDonough, F. Peerally, R. Medrano, I. Neitzel, Y. Gogotsi, and S. Osswald, Nanotechnology, 24, 205703 (2013).
8. L. Hawelek, A. Brodka, S. Tomita, J. C. Dore, V. Honkimäki, and A. Burian, Diam. Relat. Mater., 20, 1333 (2011).
9. I. Alexandrou, H. Wang, N. Sano, and G. A. J. Amaratunga, J. Chem. Phys., 120, 1055 (2004).
10. N. Sano, H. Wang, I. Alexandrou, M. Chhowalla, K. B. K. Teo, G. A. J. Amaratunga, and K. Iimura, J. Appl. Phys., 92, 2783 (2002).
11. F. Banhart, Reports on Progress in Physics, 62, 1181 (1999).
12. Y. Gao, Y. S. Zhou, J. B. Park, H. Wang, X. N. He, H. F. Luo, L. Jiang, and Y. F. Lu, Nanotechnology, 22, 165604 (2011).
13. Y. Yang, X. Liu, X. Guo, H. Wen, and B. Xu, J. Nanopart. Res., 13, 1979 (2011).
14. T. Cabioc’h, M. Jaouen, M. F. Denanot, and P. Bechet, Appl. Phys. Lett., 73, 3096 (1998).
15. M. Bystrzejewski, M. H. Rummeli, T. Gemming, H. Lange, and A. Huczko, New Carbon Mater., 25, 1 (2010).
16. M. Zhao, H. Song, X. Chen, and W. Lian, Acta Mater., 55, 6144 (2007).
17. S. Krishnamurthy, Y. V. Butenko, V. R. Dhanak, M. R. C. Hunt, and L. Šiller, Carbon, 52, 145 (2013).
18. J. K. McDonough, A. I. Frolov, V. Presser, J. Niu, C. H. Miller, T. Ubieto, M. V. Fedorov, and Y. Gogotsi, Carbon, 50, 3298 (2012).
19. S. Tomita, A. Burian, J. C. Dore, D. LeBolloch, M. Fujii, and S. Hayashi, Carbon, 40, 1469 (2002).
20. S. Tomita, T. Sakurai, H. Ohta, M. Fujii, and S. Hayashi, J. Chem. Phys., 114, 7477 (2001).
21. S. Osswald, G. Yushin, V. Mochalin, S. O. Kucheyev, and Y. Gogotsi, J. Amer. Chem. Soc., 128, 11635 (2006).
22. C. Portet, G. Yushin, and Y. Gogotsi, Carbon, 45, 2511 (2007).
23. P. Simon and Y. Gogotsi, Philos. Trans. Royal Society A: Math., Phys. and Engin. Sci., 368, 3457 (2010).
24. P. Simon and Y. Gogotsi, Nature Materials, 7, 845 (2008).
25. E. G. Bushueva, P. S. Galkin, A. V. Okotrub, L. G. Bulusheva, N. N. Gavrilov, V. L. Kuznetsov, and S. I. Moiseekov, Phys. Status Solidi B, 245, 2296 (2008).
26. E. G. Bushueva, A. V. Okotrub, P. S. Galkin, V. L. Kuznetsov, and S. I. Moseenkov, in Nanocarbon & Nanodiamond, p. 11, St. Petersburg, Russia (2006).
27. F.-D. Han, B. Yao, and Y.-J. Bai, J. Phys. Chem. C, 115, 8923 (2011).
28. H. J. Zhang, H. H. Song, J. S. Zhou, H. K. Zhang, and X. H. Chen, Acta Phys-Chim. Sin., 26, 1259 (2010).
29. W. Gu, N. Peters, and G. Yushin, Carbon, 53, 292 (2013).
30. D. Pech, M. Brunet, H. Durou, P. H. Huang, V. Mochalin, Y. Gogotsi, P. L. Taberna, and P. Simon, Nature Nanotechnol., 5, 651 (2010).
31. C. Portet, J. Chmiola, Y. Gogotsi, S. Park, and K. Lian, Electrochim. Acta, 53, 7675 (2008).
32. G. Feng, D.-E. Jiang, and P. T. Cummings, J. Chem. Theory and Computation, 8, 1058 (2012).
33. M. M. Hantel, V. Presser, J. K. McDonough, G. Feng, P. T. Cummings, Y. Gogotsi, and R. Kötz, J. Electrochem. Soc., 159, A1897 (2012).
34. P. F. Fulvio, R. T. Mayes, X. Wang, S. M. Mahurin, J. C. Bauer, V. Presser, J. McDonough, Y. Gogotsi, and S. Dai, Adv. Funct. Mater., 21, 2208 (2011).
35. J. S. Huang, B. G. Sumpter, V. Meunier, G. Yushin, C. Portet, and Y. Gogotsi, J. Mater. Res., 25, 1525 (2010).
36. R. Lin, P.-L. Taberna, S. B. Fantini, V. Presser, C. R. Pérez, F. O. Malbosc, N. L. Rupesinghe, K. B. K. Teo, Y. Gogotsi, and P. Simon, J. Phys. Chem. Lett., 2, 2396 (2011).



 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
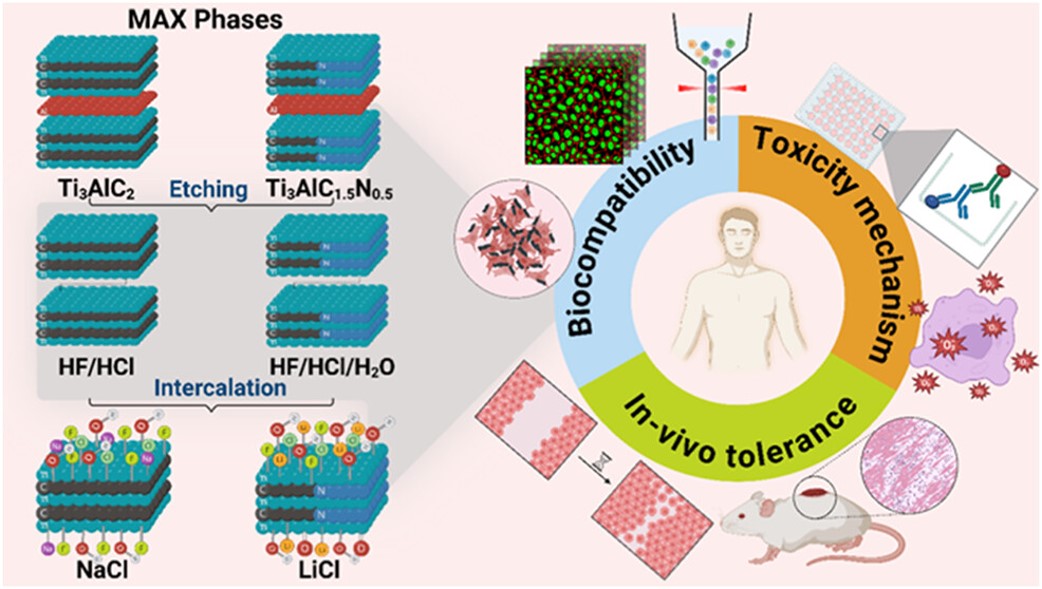 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.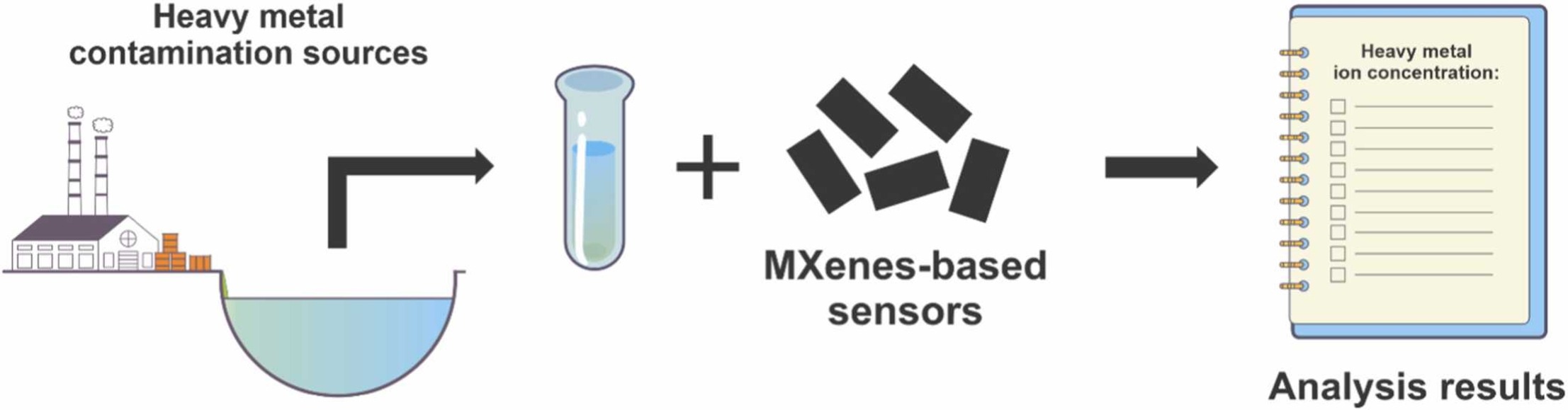 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!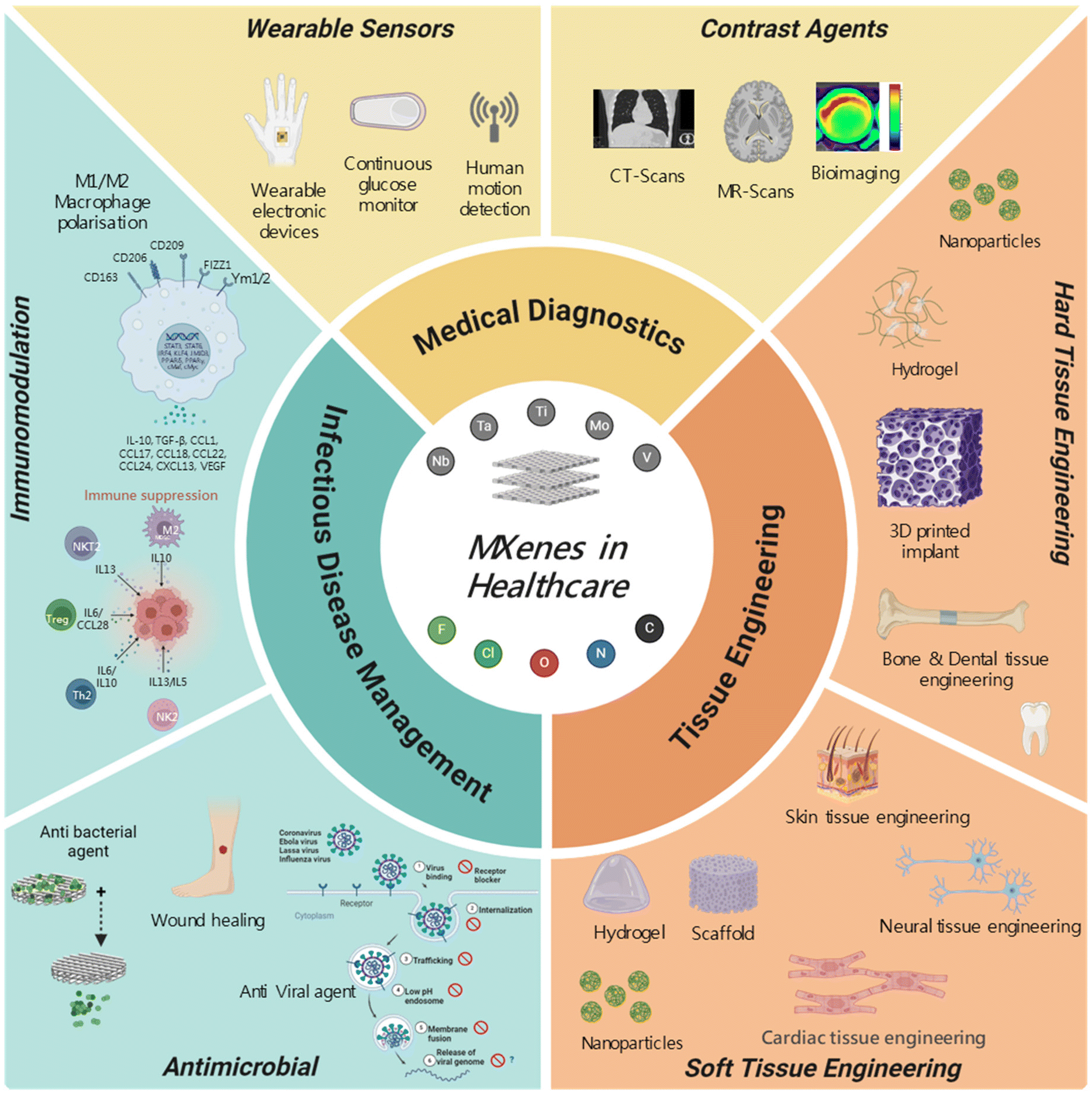 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.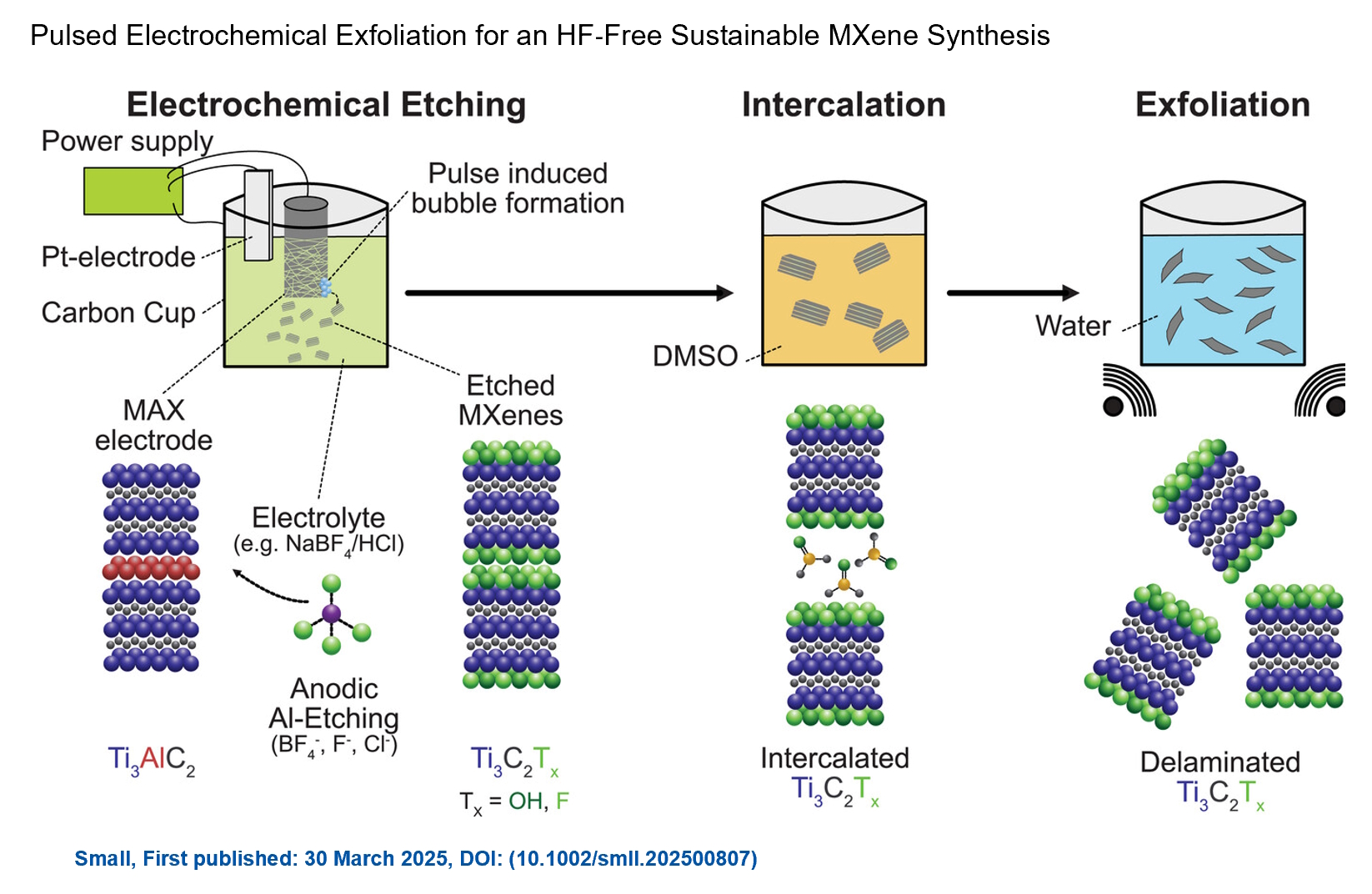 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.