| Rus | Eng |
More than twenty 2D carbides, nitrides and carbonitrides of transition metals (MXenes) have been synthesized and studied, and dozens more predicted to exist. Highly electrically conductive MXenes show promise in electrical energy storage, electromagnetic interference shielding, electrocatalysis, plasmonics and other applications.
It’s been just over five years since researchers in Drexel’s Department of Materials Science and Engineering reported on a new, two-dimensional material composed of titanium and carbon atoms.  Their creation, dubbed MXene, which is, cleverly, both chemical shorthand for the material and a homonym of Maxine, is now the focus of research at dozens of partner institutions from countries spanning every continent but Antarctica. And a feature story, on the cover of the latest edition of Nature Reviews Materials, suggests that even more should take up the pursuit.
Their creation, dubbed MXene, which is, cleverly, both chemical shorthand for the material and a homonym of Maxine, is now the focus of research at dozens of partner institutions from countries spanning every continent but Antarctica. And a feature story, on the cover of the latest edition of Nature Reviews Materials, suggests that even more should take up the pursuit.
MXene entered the conversation, in the midst of a flurry of research around the idea that atom-thick materials could be the key to building smaller, faster electronics; improving energy storage devices; adding impressive durability to products from tennis rackets to military equipment; and even repairing damaged neurons. Most of this excitement stemmed from the discovery of the amazing properties of two-dimensional graphite — called graphene — and the Nobel Prize awarded to the researchers at the University of Manchester who first reported them.
The initial excitement about graphene as the next “miracle material” with “very attractive properties” — which would put it in a category with things like aluminum, steel and plastic — has been tempered in recent years, because it has not found many real-world applications thus far, despite huge investments into research that resulted in more than 60,000 papers being published about it since 2004. But MXene is one of its contemporaries that is still being explored for many of the same uses, and already showing better performance in some of them.
“MXenes are a worthy research subject because there are so many MXenes and they have already shown unique properties, outperforming other materials in several applications,” said Babak Anasori, PhD, a research assistant professor in the A.J. Drexel Nanomaterials Institute and the lead author of the Nature Reviews Materials article.
MXene shows tremendous potential for use in energy storage devices, according to Yury Gogotsi, PhD, the principal investigator on the project that led to MXene’s discovery, who is the Distinguished University and Trustee Chair Professor at Drexel and director of the Nanomaterials Institute. Gogotsi’s team has published extensively on the material’s ability to hold and discharge electricity at exceptional rates without deteriorating. This is one area where MXene has a clear leg up on its forerunner, because its conductive properties can be controlled and have even proven to be highly tunable. And while graphene displays unparalleled electrical conductivity, it is difficult to control the torrential flow of current through it — which is a necessity for any material with aspirations of being used in electronic devices.
Gogotsi’s research also suggests that MXene has surpassed the impressive conductivity of graphene when both are scaled up to the paper-thin form that would be used in battery electrodes. And at the other end of the spectrum, MXene in its thinnest form, which is transparent, also exhibits superior conductivity to a graphene coating. Researchers are examining the possibility of using MXene films for touch screen technology and transparent energy storage devices.

Researchers in Gogotsi’s lab have also examined MXene for use as a chemical “strainer,” of sorts. Their experiments have focused on its ability to trap other molecules and ions in its atomic structure, while allowing others to pass, unimpeded. This property could be useful in corralling toxins in the body, as well as in our drinking water.
Layering other elements or compounds between sheets of MXene also produces promising results when it comes to enhancing the material’s durability. Inserting a polymer generates a version of the material that could be used for flexible electrodes in energy storage and wearable technology.
Most recently, the group has published on MXene’s ability to block another pesky intruder: electromagnetic radiation. The discovery that MXene can ward off electromagnetic interference from our ubiquitous, mobile devices seems to hold a great deal of promise because a thin layer of MXene (10 times thinner than a sheet of paper) is extremely effective as a spray coating that could be applied to individual components inside the devices.
 One of the most intriguing aspects in the pursuit of understanding how this material can be used is that it can be made with quite a variety of atoms. To date, MXene researchers have produced about two dozen distinct MXenes — moving on from the original titanium and carbon “recipe” to produce the material with pairings that have linked most of the elements in titanium’s group (the early transition metals) with both carbon and nitrogen — resulting in MXenes with their own unique properties. And the group has identified that more than 100 other combinations can be produced as stable materials.
One of the most intriguing aspects in the pursuit of understanding how this material can be used is that it can be made with quite a variety of atoms. To date, MXene researchers have produced about two dozen distinct MXenes — moving on from the original titanium and carbon “recipe” to produce the material with pairings that have linked most of the elements in titanium’s group (the early transition metals) with both carbon and nitrogen — resulting in MXenes with their own unique properties. And the group has identified that more than 100 other combinations can be produced as stable materials.
Herein lies the research challenge offered up in the Nature Materials Reviews piece. As new MXenes are being produced and explored, researchers suggest that they will get closer to understanding the ion dynamics between layers of MXene, which will be key to using them for developing new kinds of batteries. So too will be the effort to create MXenes with uniform terminations so they can be tailored for particular uses in electronics — this is roughly equivalent to making sure every shape and size of Lego block has the same round connectors so they’re compatible with the rest of the set.
And, as with graphene, the cost of producing the material is a key determinant in whether or not it’s viable to move forward with research. Michel Barsoum, PhD, distinguished professor in Drexel’s College of Engineering, who discovered MXene along with Gogotsi, and whose research focuses on synthesizing its chemical precursor, MAX Phase, recently published about a low-cost method for churning it out, which could drop the cost of MXene well below graphene.
Each of these challenges is an important hurdle that MXene must overcome on the way to being commercially viable, according to Gogotsi. But the team has made promising strides in scaling up its production process while also improving quality control. While most nanomaterials are only available in “nano” quantities, Gogotsi’s lab can make as much as 100 grams of MXene at a time, using a reactor developed with the Materials Research Center in Ukraine. This means that one of the biggest obstacles is out of the way and, with the help of broadening research efforts, MXene could soon be a name in technology as well.

“The fact that they can be produced in 100-gram quantities in the lab is a breakthrough that clearly shows that their practical applications are real,” Gogotsi said.
Since 2011, Gogotsi’s team has received just over $1 million to support its MXene research from funders that include the U.S Department of Energy, Oak Ridge National Laboratory and King Abdullah University of Science and Technology in Saudi Arabia. To ensure its commercial viability, when the time comes, Drexel has done extensive work to protect the intellectual property related to MXene, which covers compositions of matter, applications and methods of manufacture. According to Elizabeth Poppert, PhD, the licensing manager in Drexel’s Office of Technology Commercialization who handles MXene’s IP, its portfolio currently includes three issued patents that cover broad composition of matter claims, with 10 additional pending international and U.S. patent applications.
Source: https://newsblog.drexel.edu
RELATED ARTICLES:
Cleaning up electromagnetic pollution by containing the emissions with a thin coating of a nanomaterial called MXene




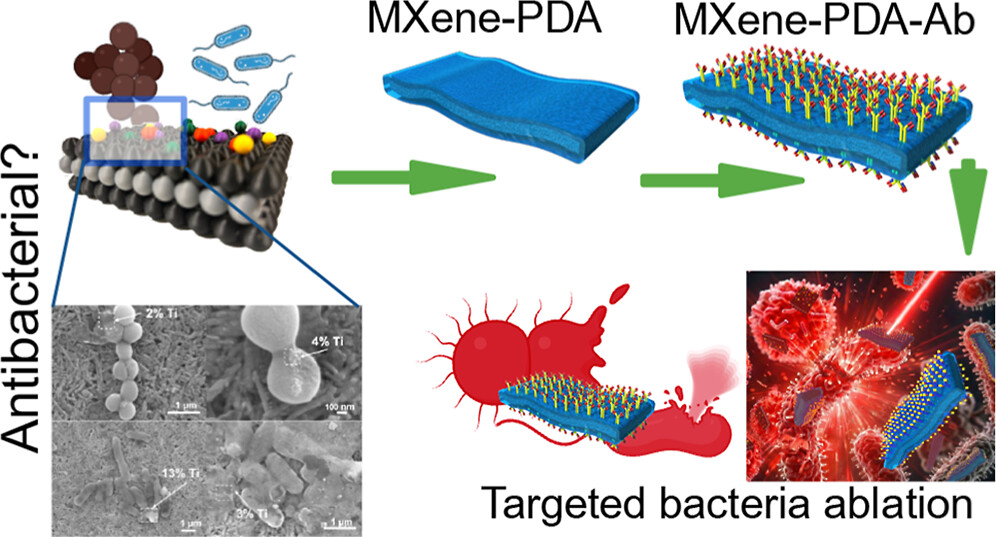 Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy.
Do MXene nanosheets possess intrinsic antibacterial activity? A systematic study of high-quality Ti-, V-, and Nb-based MXenes reveals negligible inherent antimicrobial effects while highlighting their strong potential for targeted photothermal antibacterial therapy. Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.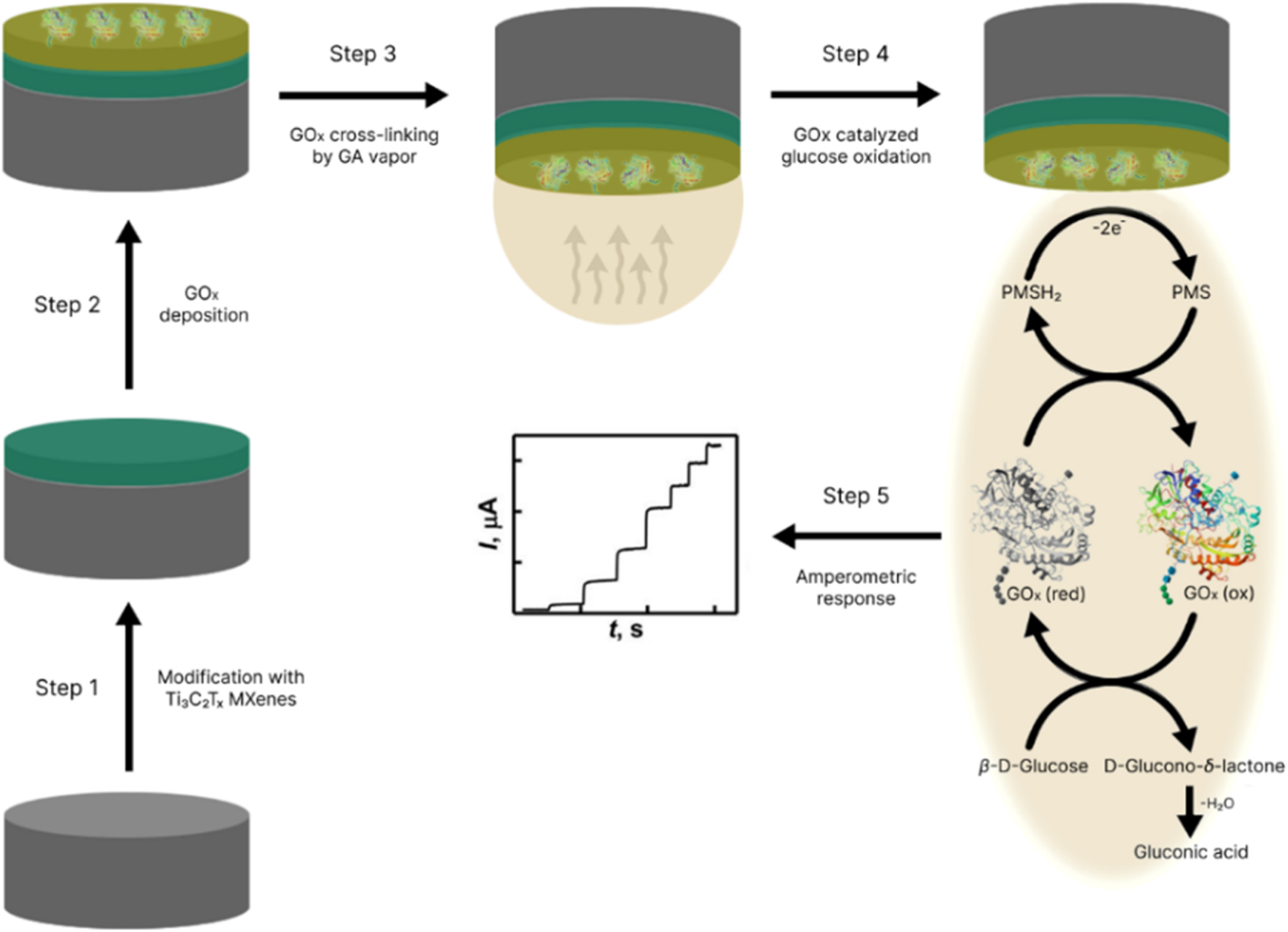
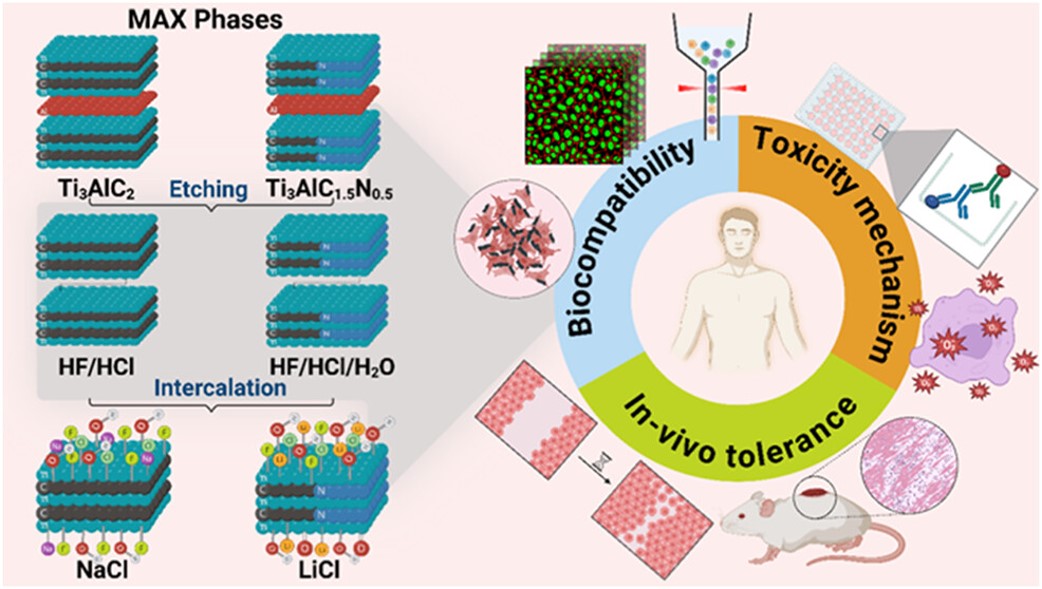 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.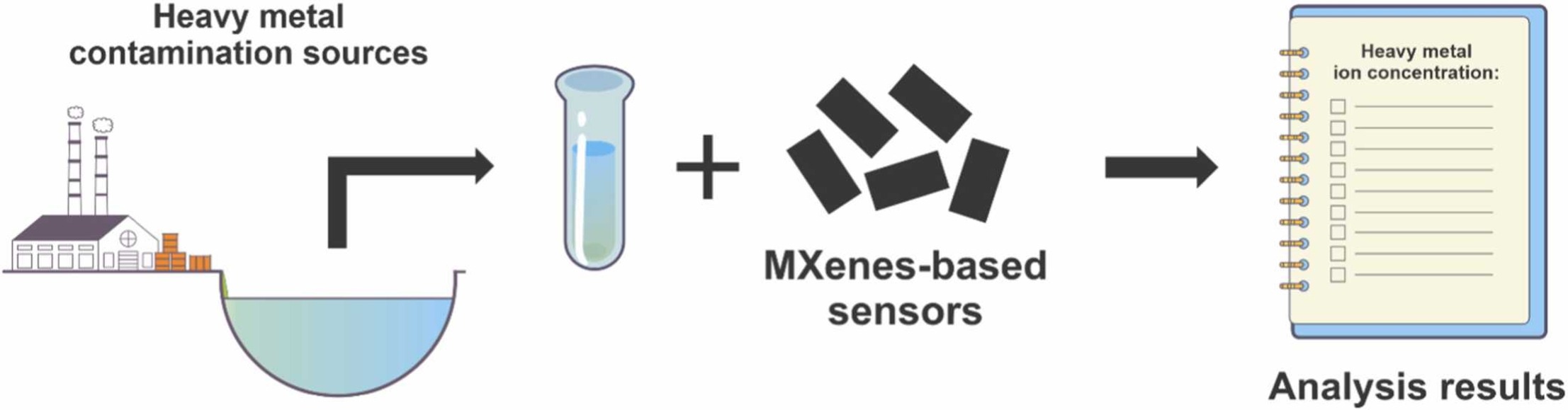 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!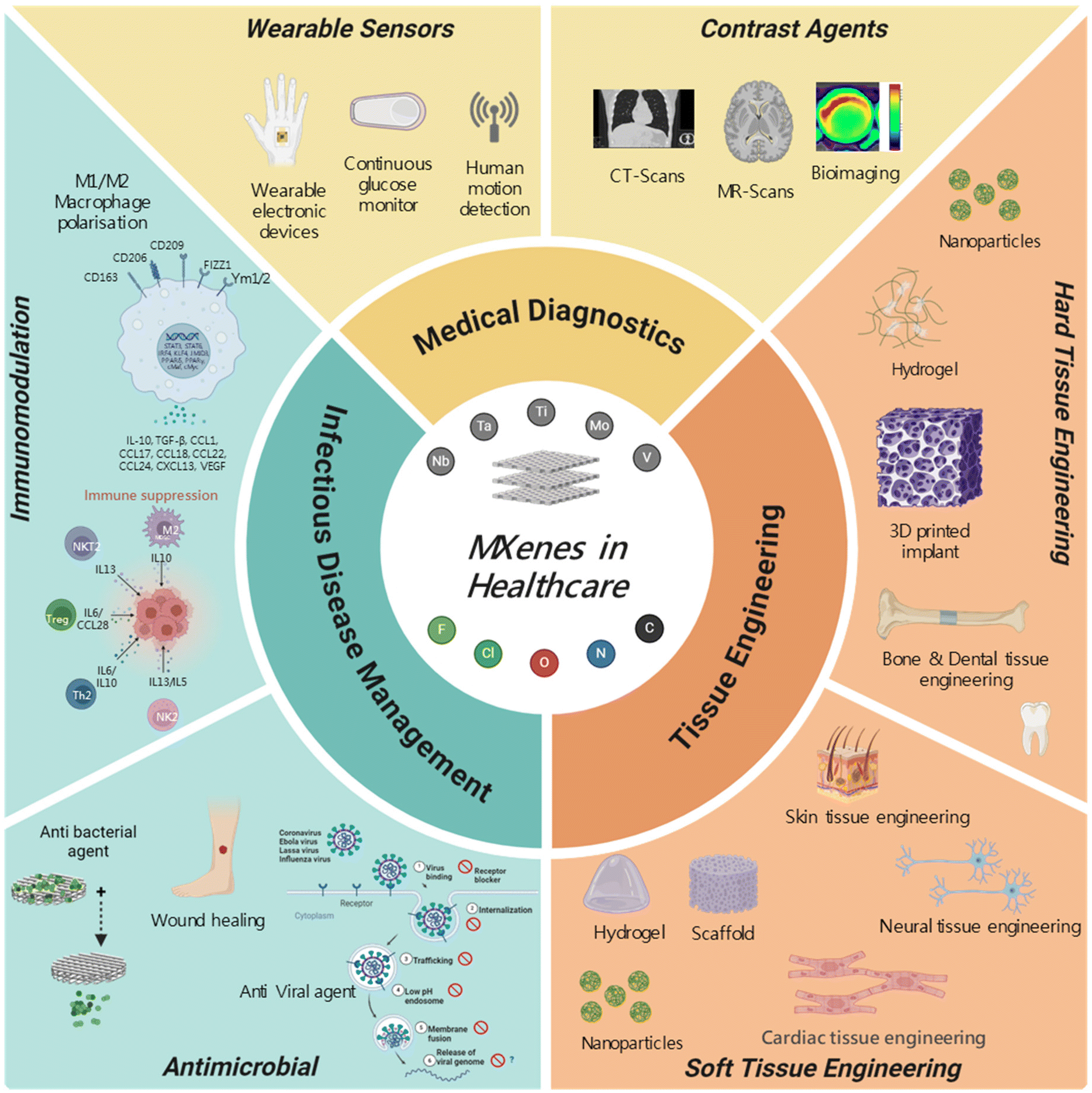 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.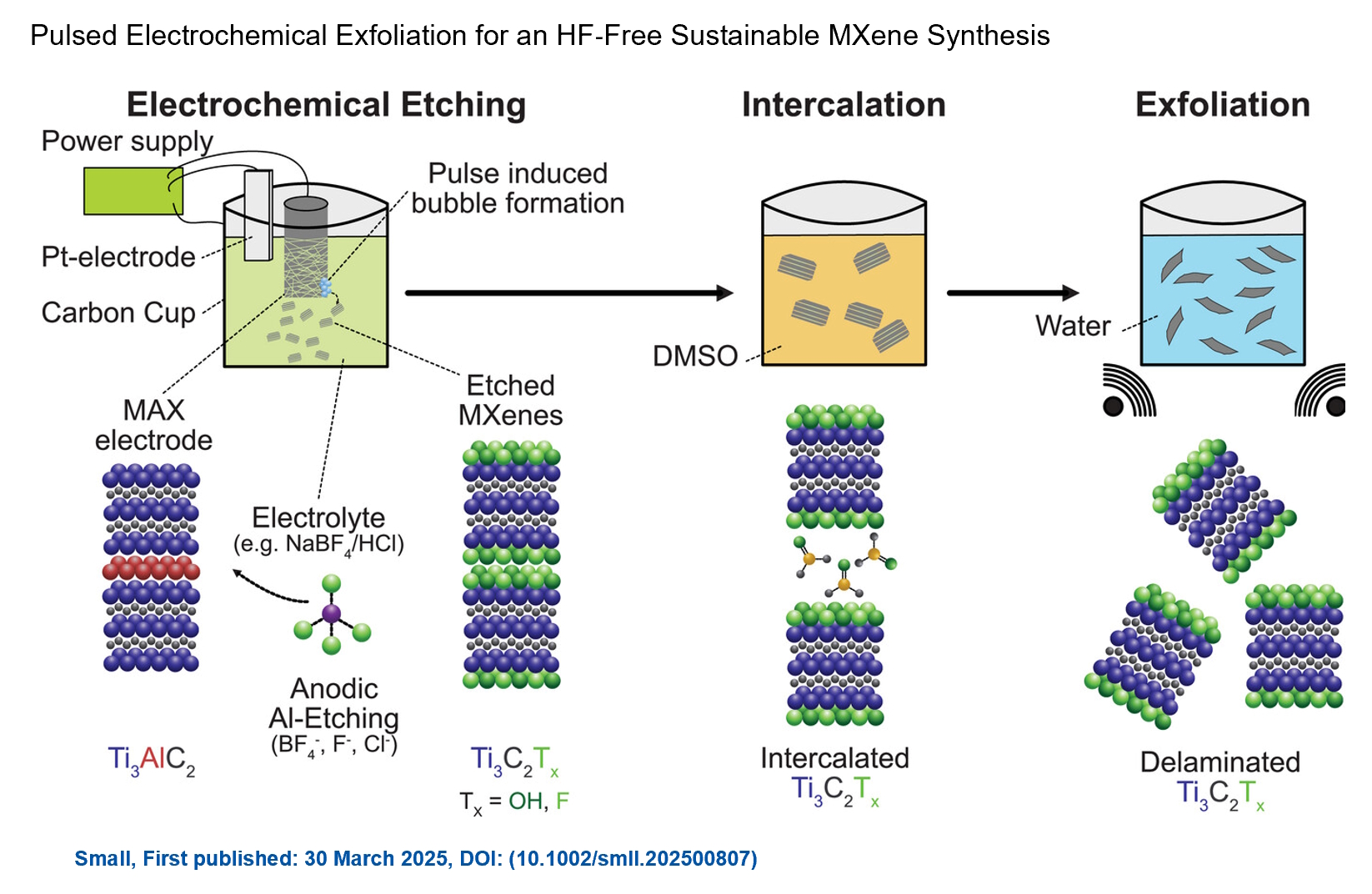 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.