| Rus | Eng |
Liquid Benzene Squeezed to Form Diamond Nanothreads
The classic Beatles song “Lucy in the Sky with Diamonds” may have a new meaning. Scientists announced they have likely discovered the strongest, stiffest diamond-based nanomaterial to date. Its properties suggest it could have important industrial applications, such as in transportation or aerospace manufacturing, and it might revive the idea of building elevators to space.
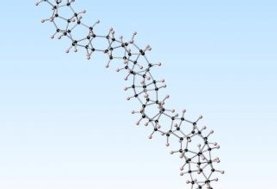 A team led by chemist John Badding of Pennsylvania State University took an approach reminiscent of the way Superman squeezed coal into diamond in comic books. The researchers found that isolated, liquid-state benzene molecules, which consist of rings of carbon atoms, assemble into surprisingly neat and orderly chains after enduring slow, alternating cycles of pressure. The resulting thread, merely three atoms across and thousands of times thinner than a strand of hair, appears to have a zigzagging arrangement of rings of carbon atoms in the shape of a triangular pyramid—a formation similar to diamond’s. Such a structure, which scientists didn’t know was possible until now, could be the strongest and most durable nanomaterial ever made.
A team led by chemist John Badding of Pennsylvania State University took an approach reminiscent of the way Superman squeezed coal into diamond in comic books. The researchers found that isolated, liquid-state benzene molecules, which consist of rings of carbon atoms, assemble into surprisingly neat and orderly chains after enduring slow, alternating cycles of pressure. The resulting thread, merely three atoms across and thousands of times thinner than a strand of hair, appears to have a zigzagging arrangement of rings of carbon atoms in the shape of a triangular pyramid—a formation similar to diamond’s. Such a structure, which scientists didn’t know was possible until now, could be the strongest and most durable nanomaterial ever made.
Badding says that the team’s discovery was serendipitous: “Honestly, it was just an accident.” Thomas Fitzgibbons, a graduate student in Badding’s lab, wanted to study materials made by the organic chemical compound benzene. When isolated, benzene molecules can react in interesting ways to form unique structures. To study these structures using conventional techniques, however, Fitzgibbons needed large quantities of the product. He brought a sample of liquid benzene to a machine called a Paris-Edinburgh device at Oak Ridge National Laboratory in Tennessee and put the molecules into a high-pressure cell. In general, when a liquid is squeezed under intense pressure, it transforms into a solid. “It essentially freezes,” Badding says. Once frozen, benzene molecules align into predictable patterns of stacked columns.
What happened next is the unusual part. Scientists have generally believed that as compression continues, the benzene molecules eventually yield a sloppy, white powder. “People thought they’d react in a disorganized way and make a mess,” Badding says.
But instead of disarray, Fitzgibbons saw order. “That was a shock to us, to say the least,” Badding confesses. The researchers were so surprised, they deployed a battery of techniques to confirm the finding, including x-ray and neutron diffraction, transmission electron microscopy and vibrational spectroscopy. Their results were consistent: they saw order.
The reason for this unexpected alignment of benzene molecules may lie in the timing of the compression. Scientists generally create benzene materials in small amounts by quick cycles of pressure changes. To make more product, the compression cycles must be slower. “It seems we gave benzene molecules time to arrange into a pattern, particularly nanothreads,” Badding says. This slow compression was key to their discovery.
Yury Gogotsi, director of the A. J. Drexel Nanomaterials Institute at Drexel University, says that although the results are indeed exciting, he would like further confirmation and analysis of the material, for example using “much higher-resolution images, which can further shed light on the material’s structure,” Gogotsi says. “Assuming their interpretation is correct, which there’s good reason to believe, I think this discovery is significant.”
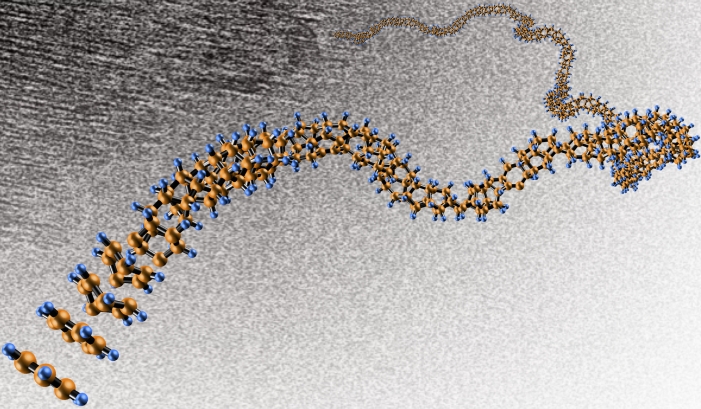 Before the nanothreads can be used commercially, Badding wants to determine their properties and behavior in different conditions and to understand exactly how the benzene molecules link up. The studies could take years, he says. Then engineers will need to figure out how best to mass-manufacture them and incorporate them into existing industrial infrastructure for various uses. For a start, these threads seem poised to replace carbon fiber, which is weaker and heavier, in commercial products such as bicycle frames, golf clubs and airplane bodies.
Before the nanothreads can be used commercially, Badding wants to determine their properties and behavior in different conditions and to understand exactly how the benzene molecules link up. The studies could take years, he says. Then engineers will need to figure out how best to mass-manufacture them and incorporate them into existing industrial infrastructure for various uses. For a start, these threads seem poised to replace carbon fiber, which is weaker and heavier, in commercial products such as bicycle frames, golf clubs and airplane bodies.
Even further in the future, the nanothreads could perhaps stretch into space to deliver supplies to the International Space Station or interact with orbiting satellites. Seriously. Futurists have long imagined that a cable anchored on Earth and attached to a satellite in orbit could be the basis for a space elevator, but making a cable long and strong enough to resist the high-altitude winds and to ferry loads safely has proved a challenge. Conventional steel cables would break under their own weight. Diamond nanothreads could in principle be both light enough and tough enough to do the job.
Even if this particular nanothread proves incapable of sending supplies or humans into orbit, its discovery could pave the way for better alternatives. This is not the first time scientists have spawned diamond structures by tricking rings of carbon into unique configurations. Diamond-like carbons, also called amorphous carbons, are typically applied as coatings to other materials, such as the protective layer on a stainless steel pan. Gogotsi says that although hitting upon a new structure is surprising and interesting, this research reminds chemists that the discovery of other similar structures is not far off. “This group has shown that it’s another member of the family of diamond structures, and I’m sure that it’s not the last,” Gogotsi says. If so, then someday a space elevator may exist, and there might really be diamonds in the sky.
Source: http://www.scientificamerican.com/article/liquid-benzene-squeezed-to-form-diamond-nanothreads/



 Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
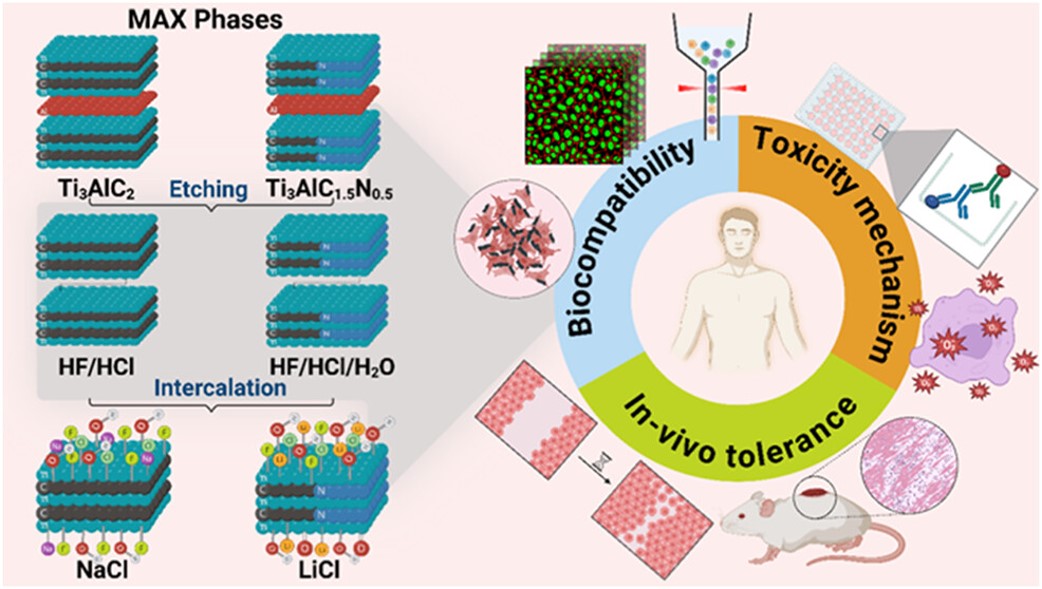 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.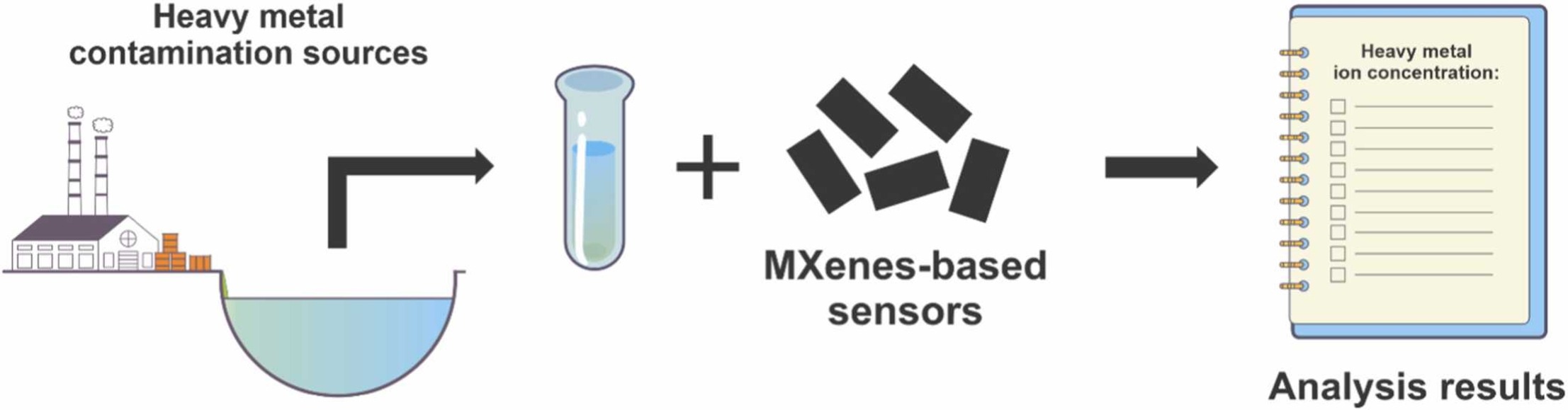 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!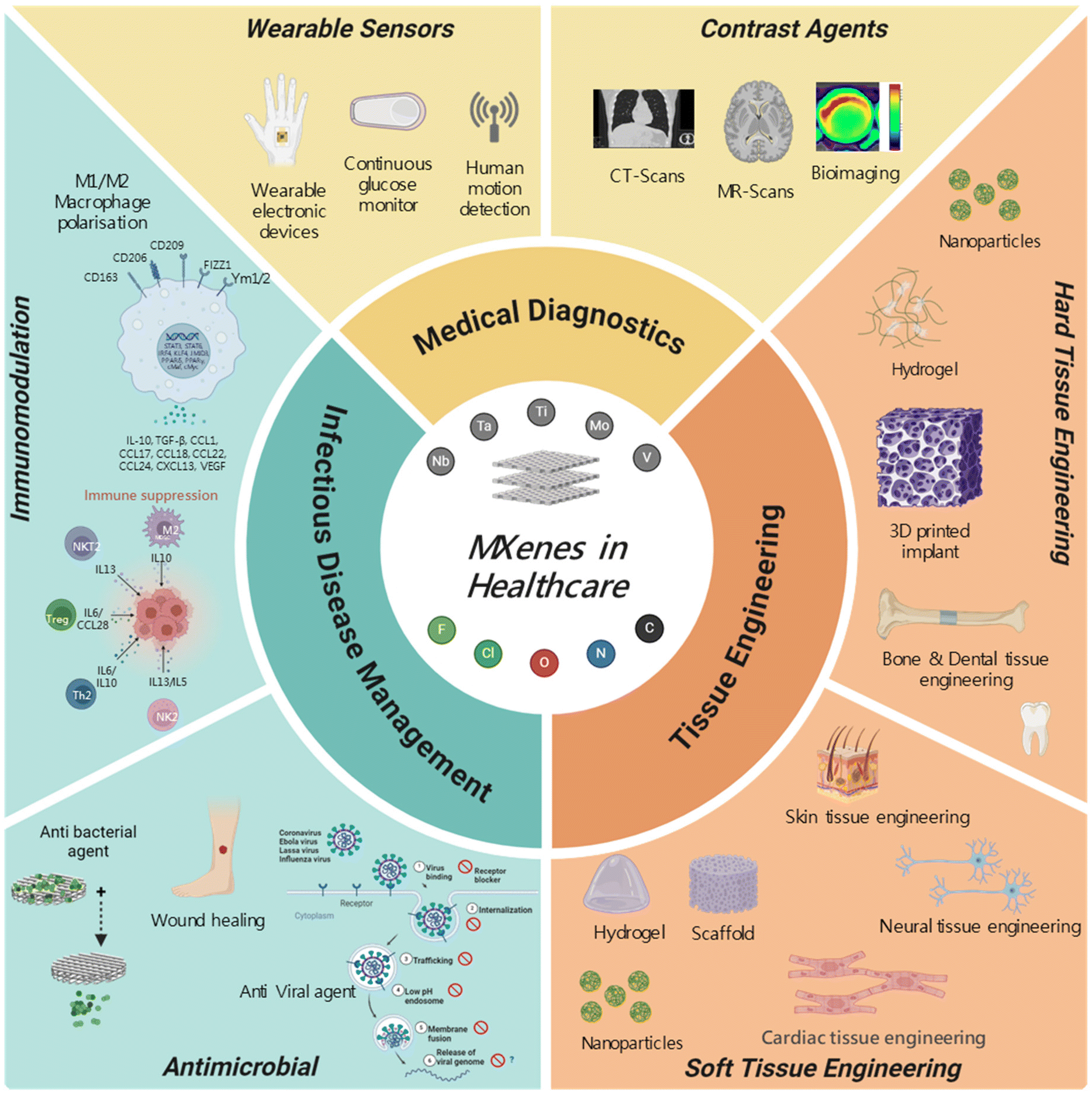 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.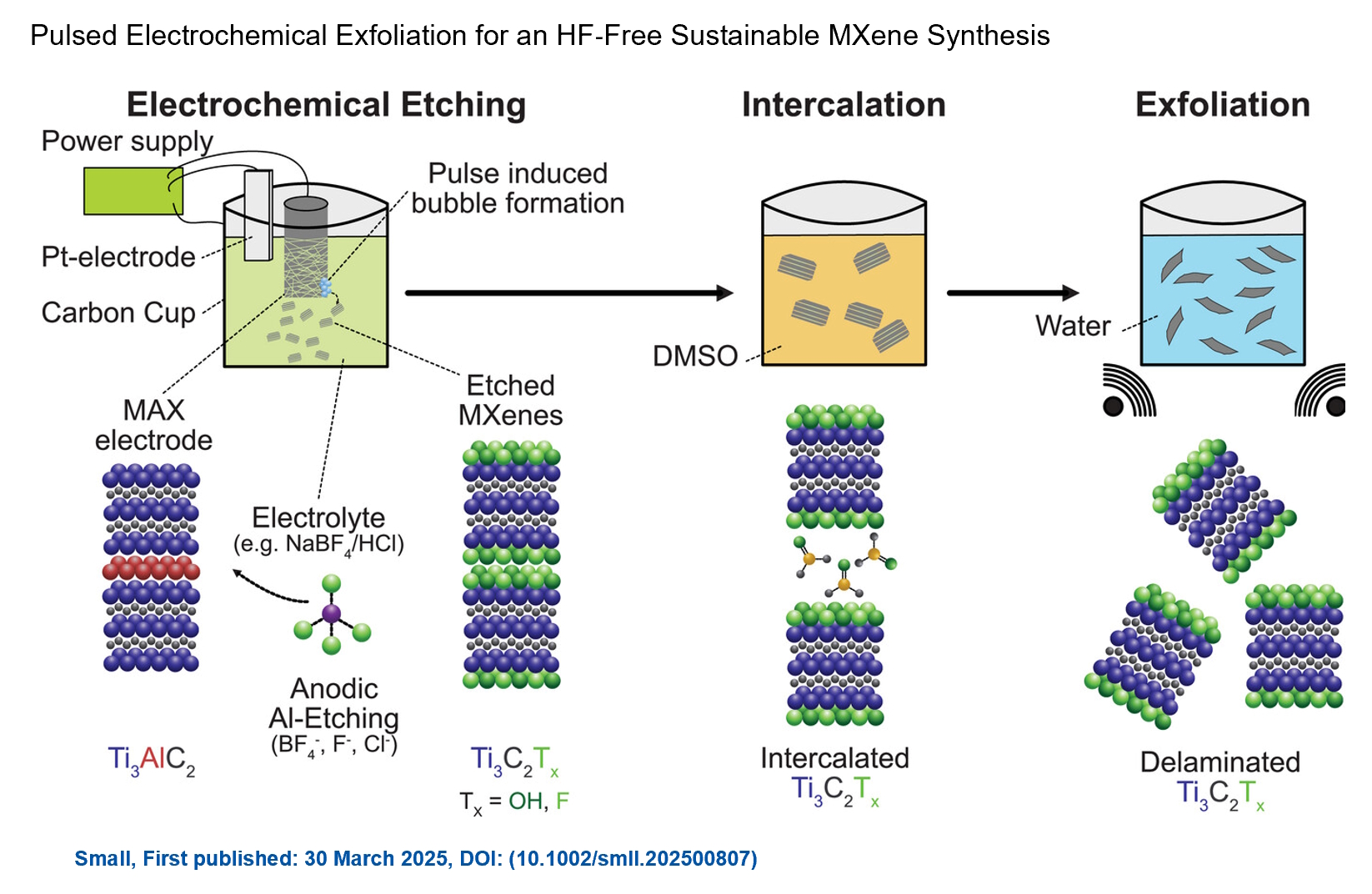 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved. Congratulations to all collaborators with this interesting joint work!
Congratulations to all collaborators with this interesting joint work!