| Rus | Eng |
Synthesis of carbon films by electrochemical etching of SiC with hydrofluoric acid in nonaqueous solvents*
a, a, a, b, Этот e-mail адрес защищен от спам-ботов, для его просмотра у Вас должен быть включен Javascript , a,
a Promotion Centre for Global Materials Research (PCGMR), Department of Material Science and Engineering, National Cheng Kung University, Tainan, Taiwan
b Department of Materials Science and Engineering, and A. J. Drexel Nanotechnology Institute, Drexel University, Philadelphia, PA 19104, USA
*In Press, Accepted Manuscript, Available online 24 January 2014, http://dx.doi.org/10.1016/j.carbon.2014.01.028
Abstract
Carbon films on SiC have many applications, ranging from tribology to electrical energy storage. Formation of epitaxial or heteroepitaxial layers of carbon on SiC by “soft solution process,” such as electro- or photochemical ones, are attractive for various fields of application, decreasing the energy consumption and making the process compatible with electronic device fabrication. We have demonstrated formation of a carbon layer on SiC ceramics by electrochemical etching in a nonaqueous electrolyte. The selective etching of Si from SiC in a single step reaction with hydrofluoric acid (HF) in different organic solvents has been carried out and the role of polarity, surface tension, density, and viscosity of the organic solvents in the formation of the carbon layer has been investigated. The solution of 1:4.6 ratio HF and ethanol at low current densities (10 and 20 mA/cm2) allows the best control over selective etching of Si forming amorphous and ordered carbon on the SiC surface. The presence of an intense G band of graphitic carbon in Raman spectra and high resolution transmission electron microscopy analysis indicate formation of ordered carbon on the surface of SiC. X-ray diffraction shows that the etching rate of α-SiC is much higher when compared to β-SiC.
|
|
|
|
|
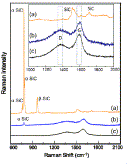
Fig. 1. Raman spectra of unetched and etched SiC. (a) Unetched SiC (b) Etched at 10 mA/cm2, (c) Etched at 20 mA/cm2. Note: G = graphite band; D = disorder induced band; Inset shows the magnified carbon range of Raman spectra of samples etched at 10 and 20 mA/cm2 current densities |
|
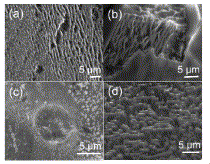
Fig. 2. SEM images of SiC etched with HF solutions in different solvents at fixed current densities 10 mA/cm2 (a) acetonitrile (b) acetone (c) water (d) isopropanol |
|
|
|
|
|
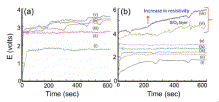
Fig. 3. (a) Voltage vs time diagram for SiC etched with HF solution in (i) water, (ii) alcohol, (iii) acetone, (iv) isopropanol, and (v) acetonitrile. (b) Voltage vs time diagram for SiC etched with 5 molar HF solution in ethanol at (i) 2.5 mA/cm2, (ii) 5.0 mA/cm2, (iii) 10 mA/cm2, (iv) 20 mA/cm2, (v) 40 mA/cm2, (vi) 60 mA/cm2, and (vii) 80 mA/cm2 |
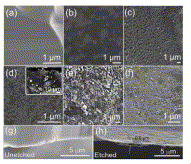
Fig. 4. SEM images of SiC etched in HF solution in ethanol at different current densities (a) unetched SiC (b) 5 mA/cm2 (c) 10 mA/cm2 (d) 20 mA/cm2 (e) 40 mA/cm2 (f) 60 mA/cm2. Note: Fig. 4 (d) inset shows a backscattered electron image of SiC etched at 20 mA/cm2. SEM micrographs of unetched SiC (g) and etched SiC (h) (at 20 mA/cm2) |
|
|
|
|
|
|
Fig. 5. TEM images of SiC etched in HF-ethanol (20 mA/cm2) produced at 200 kV |
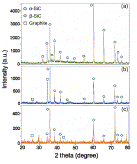
Fig. 6. XRD patterns of unetched and etched SiC (a) unetched SiC (b) etched at 10 mA/cm2 (c) etched at 20 mA/cm2. |
|
|
|
||
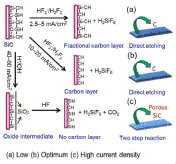
| Fig. 7. Proposed reaction mechanism of SiC etching with HF in (5 M) ethanol solution at different current densities: (a) Si reacts with HF2- and follows the single-step mechanism at low current density (b) Si reacts with HF2- and follows single step mechanism at the optimum current density (c) Si reacts with OH- follows the two-step mechanism at high current density. | ||
Source: www.sciencedirect.com




 Highlights
Highlights We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development.
We are excited to share that our Carbon-Ukraine (Y-Carbon LLC) company participated in the I2DM Summit and Expo 2025 at Khalifa University in Abu-Dhabi! Huge thanks to Research & Innovation Center for Graphene and 2D Materials (RIC2D) for hosting such a high-level event.It was an incredible opportunity to meet brilliant researchers and innovators working on the next generation of 2D materials. The insights and energy from the summit will definitely drive new ideas in our own development. Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments.
Carbon-Ukraine team had the unique opportunity to visit XPANCEO - a Dubai-based deep tech startup company that is developing the first smart contact lenses with AR vision and health monitoring features, working on truly cutting-edge developments. Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
Our Carbon-Ukraine team (Y-Carbon LLC) are thrilled to start a new RIC2D project MX-Innovation in collaboration with Drexel University Yury Gogotsi and Khalifa University! Amazing lab tours to project collaborators from Khalifa University, great discussions, strong networking, and a wonderful platform for future collaboration.
 MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.
MXenes potential applications include sensors, wound healing materials, and drug delivery systems. A recent study explored how different synthesis methods affect the safety and performance of MXenes. By comparing etching conditions and intercalation strategies, researchers discovered that fine-tuning the surface chemistry of MXenes plays a crucial role in improving biocompatibility. These results provide practical guidelines for developing safer MXenes and bring the field one step closer to real biomedical applications.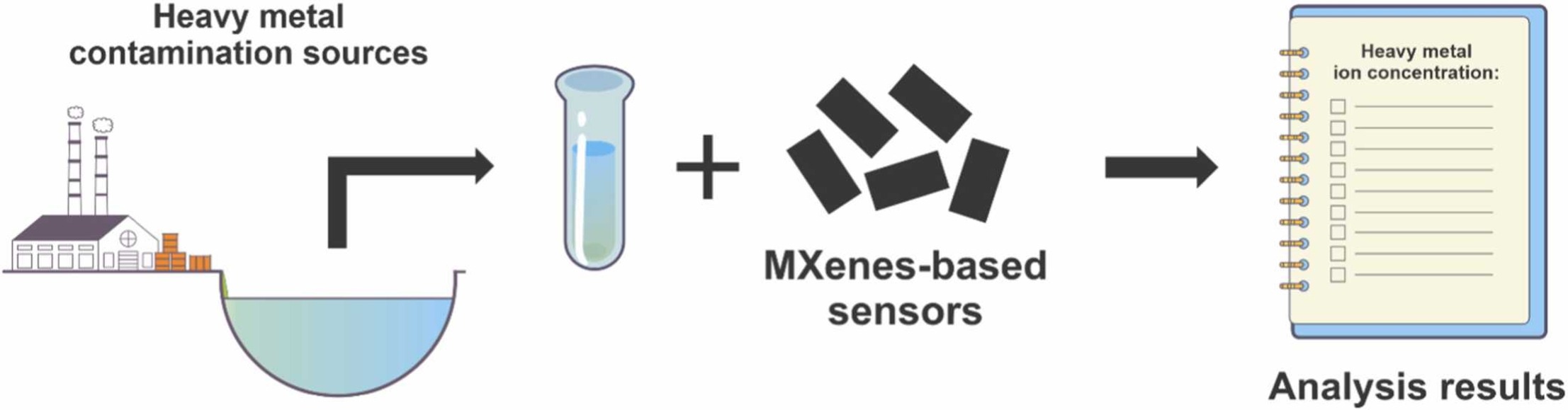 An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
An excellent review highlighting how MXene-based sensors can help tackle one of today’s pressing environmental challenges — heavy metal contamination. Excited to see such impactful work moving the field of environmental monitoring and sensor technology forward!
 Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme.
Carbon-Ukraine team was truly delighted to take part in the kickoff meeting of the ATHENA Project (Advanced Digital Engineering Methods to Design MXene-based Nanocomposites for Electro-Magnetic Interference Shielding in Space), supported by NATO through the Science for Peace and Security Programme. Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved!
Exellent news, our joint patent application with Drexel University on highly porous MAX phase precursor for MXene synthesis published. Congratulations and thanks to all team involved! Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!
Our team was very delighted to take part in International Symposium "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene event in Europe this year!  Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!
Last Call! Have you submitted your abstract for IEEE NAP-2025 yet? Join us at the International Symposium on "The MXene Frontier: Transformative Nanomaterials Shaping the Future" – the largest MXene-focused conference in Europe this year! Final Submission Deadline: May 15, 2025. Don’t miss this exclusive opportunity to showcase your research and engage with world leaders in the MXene field!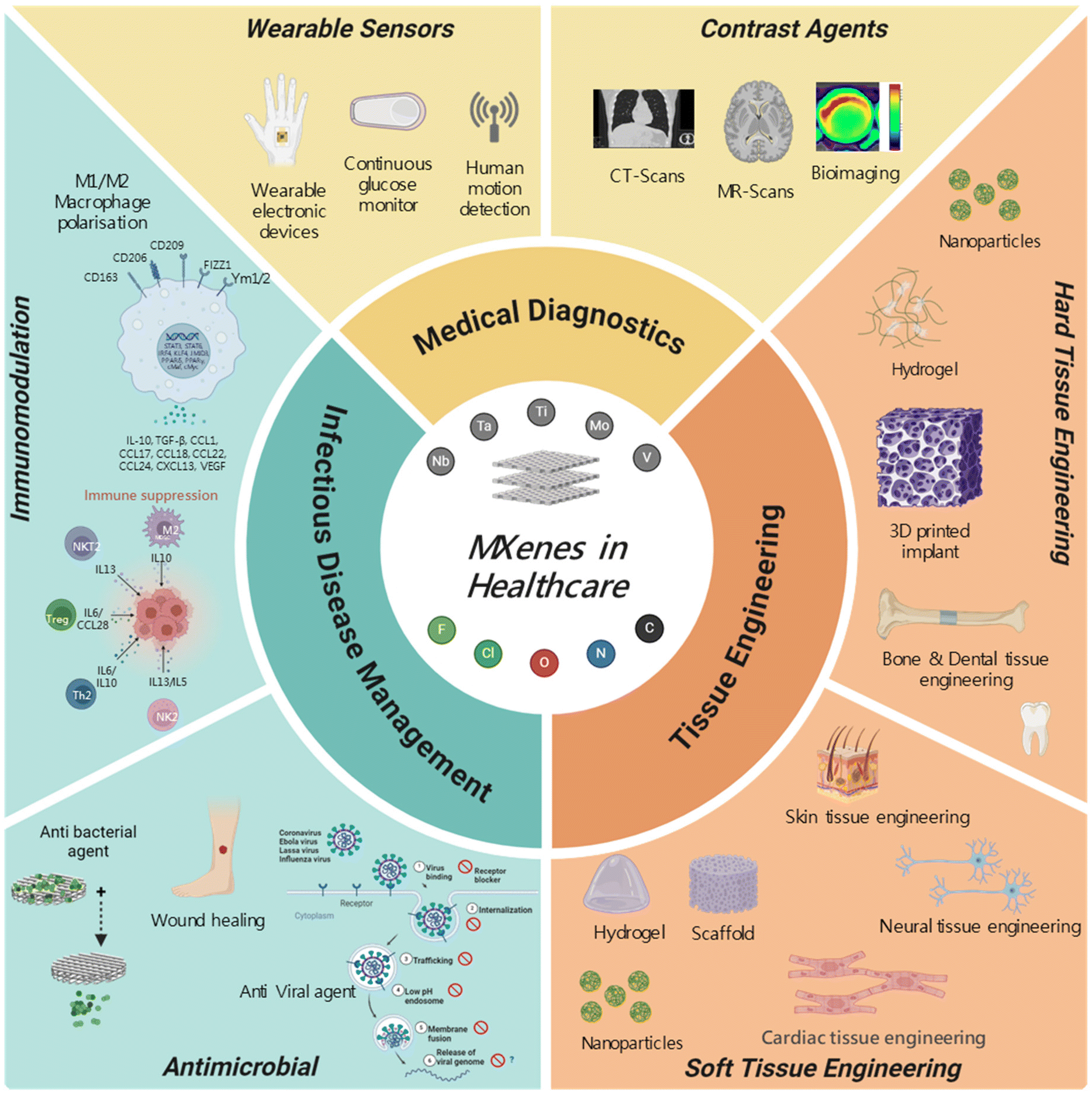 We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.
We are excited to announce the publication of latest review article on MXenes in Healthcare. This comprehensive review explores the groundbreaking role of MXenes—an emerging class of 2D materials—in revolutionizing the fields of medical diagnostics and therapeutics. Read the full article here: https://doi.org/10.1039/D4NR04853A.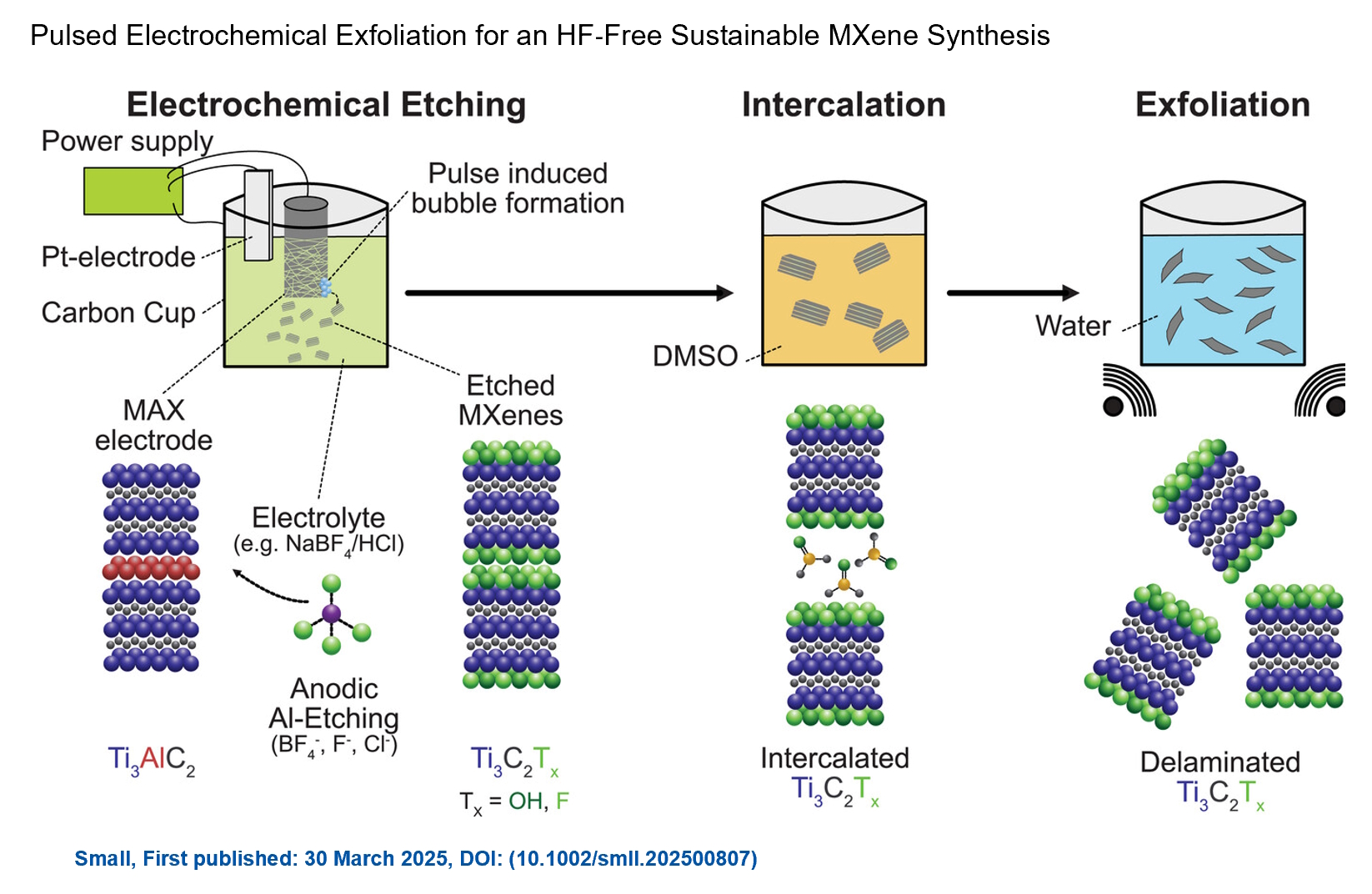 Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved.
Congratulations and thank you to our collaborators from TU Wien and CEST for very interesting work and making it published! In this work, an upscalable electrochemical MXene synthesis is presented. Yields of up to 60% electrochemical MXene (EC-MXene) with no byproducts from a single exfoliation cycle are achieved. Congratulations to all collaborators with this interesting joint work!
Congratulations to all collaborators with this interesting joint work!